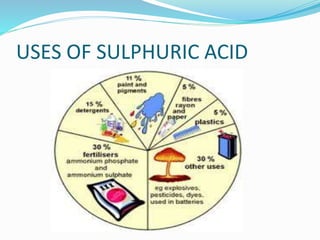
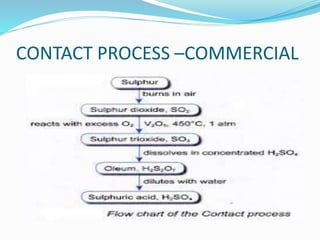
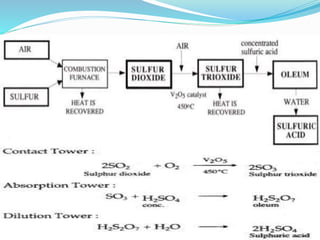
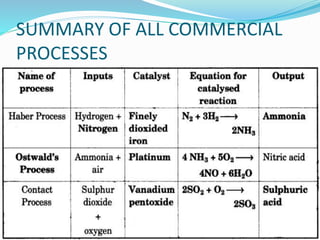
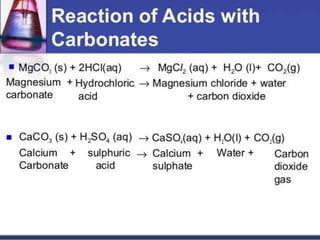
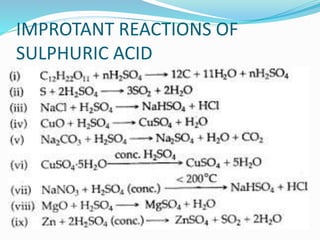
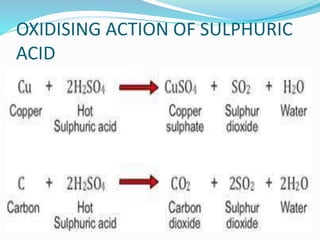
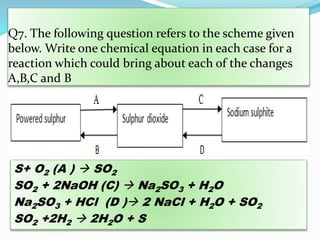
This document discusses sulphuric acid, including its production via the contact process using vanadium pentoxide as a catalyst, important properties such as being acidic, non-volatile, and an oxidizing agent. Sample reactions demonstrating these properties are given. The document also provides answers to questions about reactions of sulphuric acid such as its dehydrating effect on sugar, and how it can be used to distinguish between dilute hydrochloric acid and dilute sulphuric acid. Production of sulphuric acid and its widespread uses in industry and laboratories are covered.