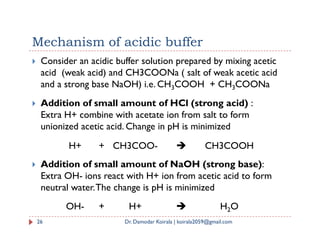
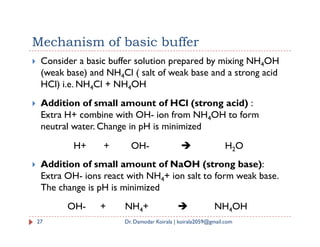
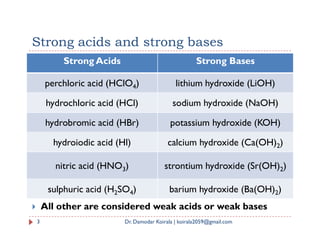
The document summarizes key concepts regarding ionic equilibrium and solubility products. It discusses four types of salt hydrolysis and how they result in acidic, basic, or neutral solutions. It then defines solubility product and solubility product expressions for different types of salts. The solubility product principle is introduced, stating that precipitation will occur when the ionic product exceeds the solubility product constant.




![Solubility product: expression
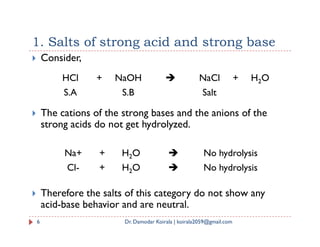
Let BA be binary salt that is sparingly soluble in water.Then,
BA(s) BA (solution) B+ + A-
Applying the law of mass action,
K = [B+] [A-]
K* [BA(s)] = [B+] [A-]
The concentration of solid salt [BA(s)] is constant. By
convention it is unity. So, [BA(s)] = 1
13 Dr. Damodar Koirala | koirala2059@gmail.com
K = [B+] [A-]
[AB(s)]](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-13-320.jpg)
![Solubility product: expression
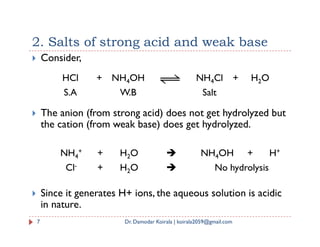
Hence, [B+] [A-] = constant = Ksp
The constant Ksp is called the solubility product of salt BA.
The above equation signifies that the product of concentration
of B+ and A- of a salt is constant independent of the individual
of B+ and A- of a salt is constant independent of the individual
concentration of B+ and A- ions when temperature is constant
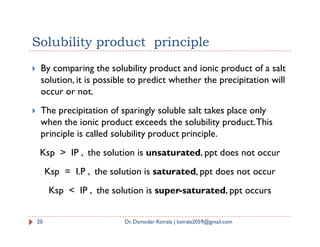
If S is the solubility of BA, then [B+] = S and [A-] = S
Ksp = [B+] [A-] = S * S = S2
Here, unit of Ksp is (mol/L)2
14 Dr. Damodar Koirala | koirala2059@gmail.com](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-14-320.jpg)
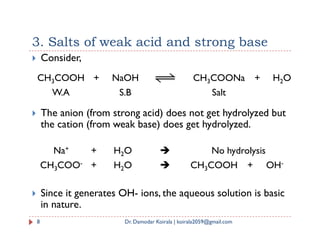
![ Salt of BA type: ( For example AgCl, CaSO4)
The solubility equilibrium can be represented as
BA(s) A-(aq) + B+(aq) and Ksp = [A-] [B+]
Let S mol/L be the solubility then
Solubility product: expression
Let S mol/L be the solubility then
[A-] = [B+] = S
Substituting the values in the expression of Ksp
Ksp = S * S = S2
Here, unit of Ksp is (Mol/L)2
15 Dr. Damodar Koirala | koirala2059@gmail.com](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-15-320.jpg)
![ Salt of BA2 type: ( For example CaF2).
The solubility equilibrium can be represented as
BA2(s) 2A-(aq) + B2+ (aq) and Ksp = [B2+] [A–]2
Let S mol/L be the solubility then
Solubility product: expression
16 Dr. Damodar Koirala | koirala2059@gmail.com
Let S mol/L be the solubility then
[B2+] = S and [A–] = 2S
Substituting the values in the expression of Ksp
Ksp = S * (2S)2 = 4 S3
Here, unit of Ksp is (Mol/L)3](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-16-320.jpg)
![ Salt of B2A type: ( For example Ag2CrO4).
The solubility equilibrium can be represented as
B2A (s) A2-(aq) + 2B+(aq) and Ksp = [B+]2 [A2-]
Let S mol/L be the solubility then
Solubility product: expression
17 Dr. Damodar Koirala | koirala2059@gmail.com
Let S mol/L be the solubility then
[B+] = 2S and [A2-] = S
Substituting the values in the expression of Ksp
Ksp = (2S)2 * S = 4 S3
Here, unit of Ksp is (Mol/L)3](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-17-320.jpg)
![ Salt of B3A2 type: ( For example Ca3(PO4)2).
The solubility equilibrium can be represented as
B3A2 (s) 2A3-(aq) + 3B2+(aq) and Ksp = [B2+]3 [A3–]2
Let S mol/L be the solubility then
Solubility product: expression
18 Dr. Damodar Koirala | koirala2059@gmail.com
Let S mol/L be the solubility then
[B2+] = 3S and [A3–] = 2S
Substituting the values in the expression of Ksp
Ksp = (3S)3 * (2S)2 = 108 S5
Here, unit of Ksp is (Mol/L)5](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-18-320.jpg)
![Solubility product: expression
Salt [A-] [B+] Ksp Ksp Ksp unit
AB S S [A-] [B+] S . S M2
A2B 2S S [A-]2 [B2+] 22. S2. S M3
19 Dr. Damodar Koirala | koirala2059@gmail.com
AB2 S 2S [A2-] [B+]2 22. S. S2 M3
A2B3 2S 3S [A3-]2 [B2+]3 22. 33. S2. S3 M5
A3B2 3S 2S [A2-]3 [B3+]2 33.22.S3.S2 M5
AxBy xS yS [Ay-]x [Bx+]y xx. yy. Sx. Sy M(x+y)](https://image.slidesharecdn.com/ionicequilibriumpart2-210528073017/85/Ionic-equilibrium-part-2-19-320.jpg)