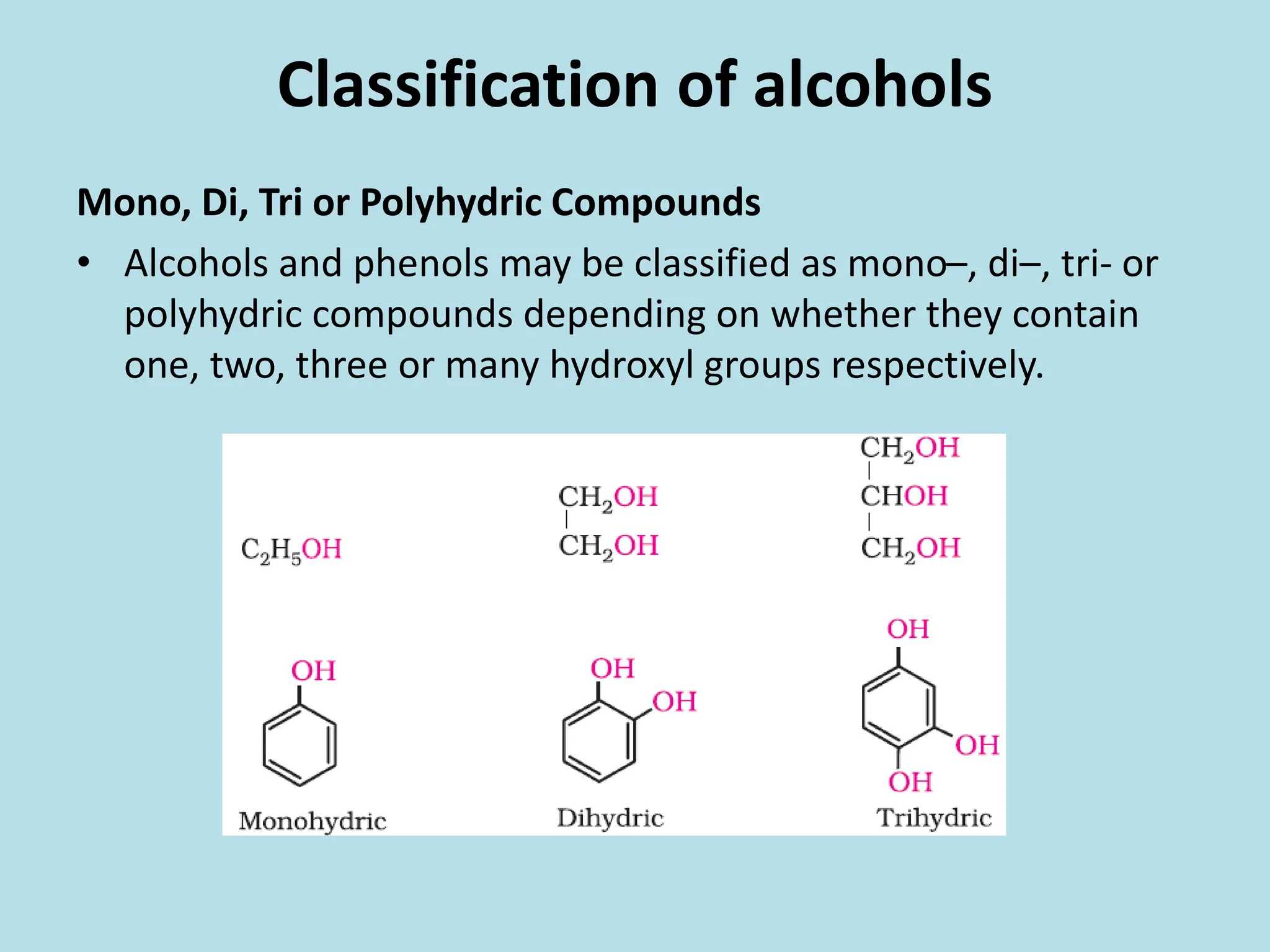
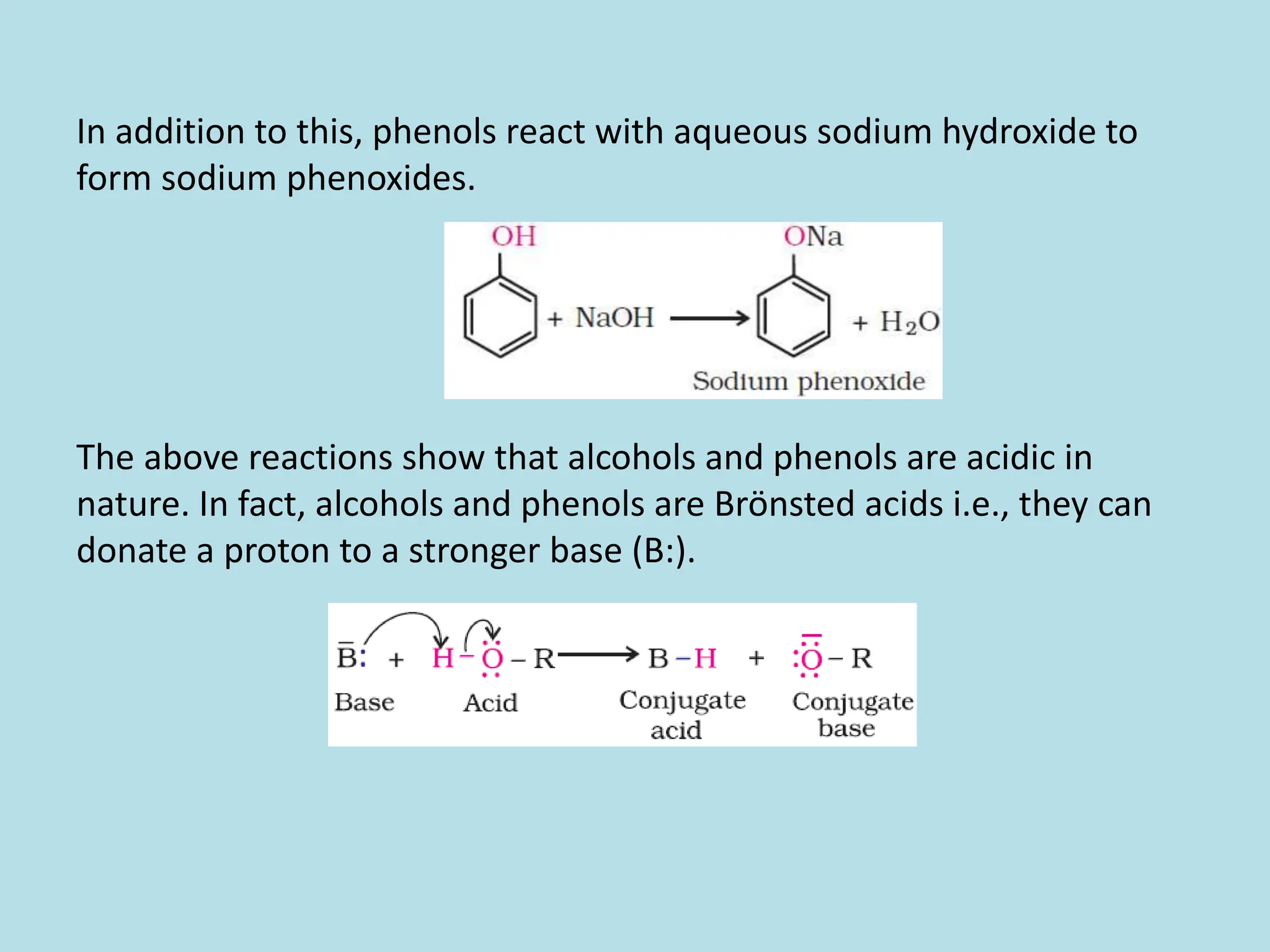
The document provides an extensive overview of alcohols, phenols, and ethers, covering their formation, classification, nomenclature, and physical properties. It discusses various methods for preparing alcohols and phenols, as well as their chemical reactions, including acidity and oxidation. Additionally, the document compares the solubility and boiling points of these compounds, highlighting their applications and significance in chemistry.