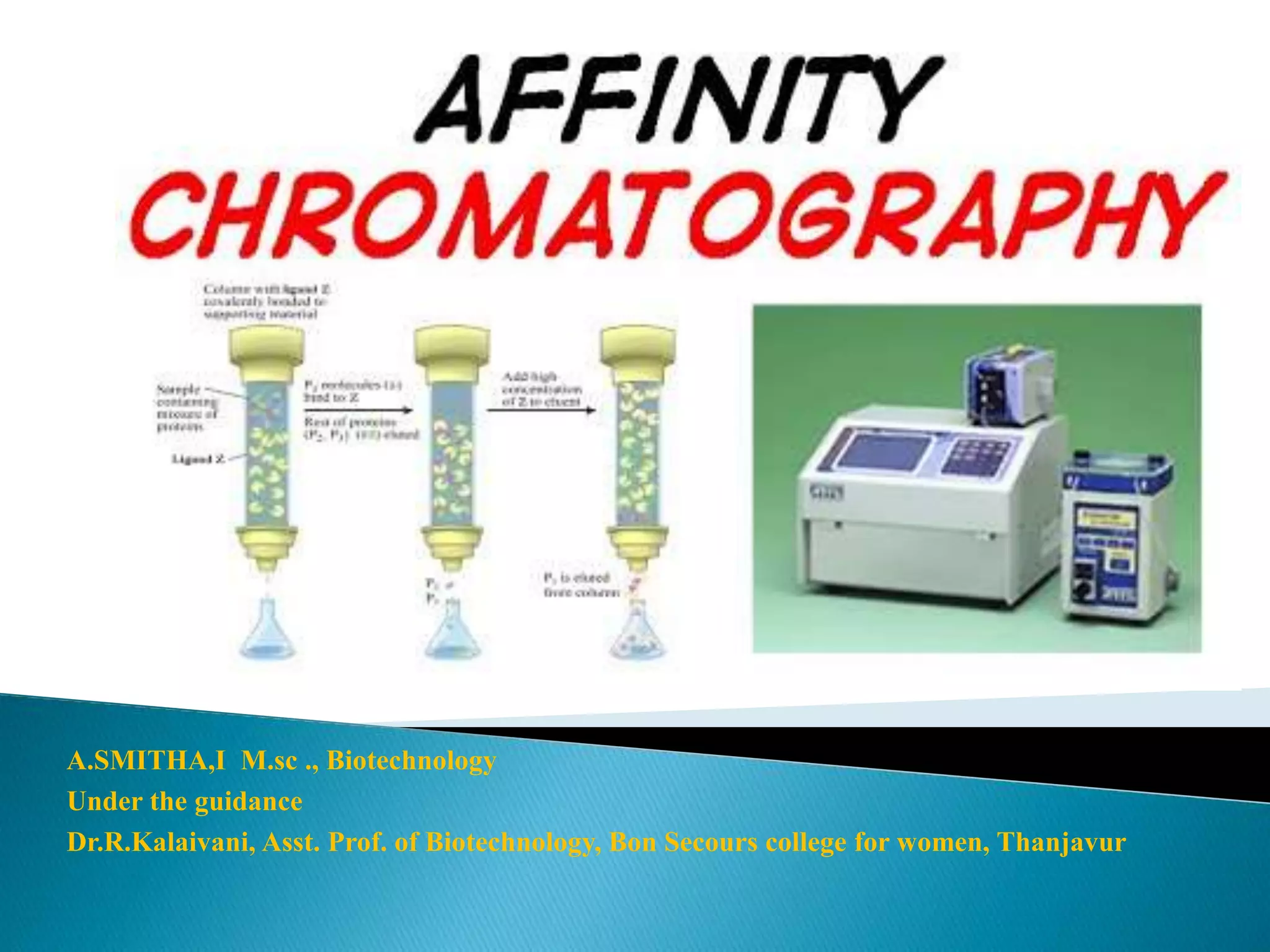
This document discusses affinity chromatography, a biophysical technique for separating and purifying biological molecules based on specific interactions between ligands and target molecules. It outlines the components of the chromatography process, including stationary phase, matrix, and ligands, as well as the procedures for sample application and elution. The document also highlights the advantages and applications of affinity chromatography, while mentioning its limitations such as time consumption and high costs.