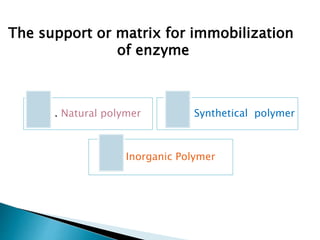
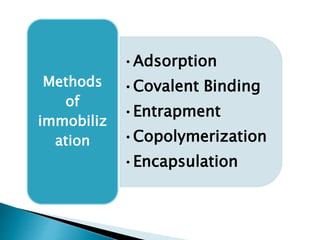
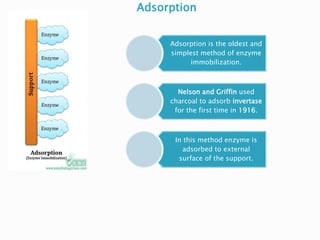
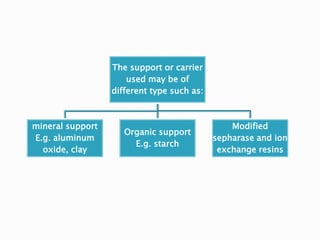
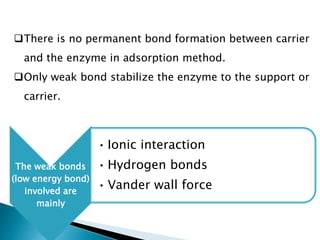
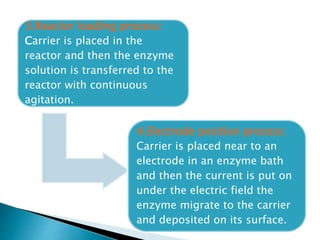
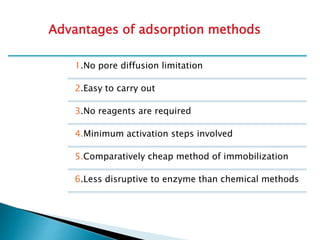
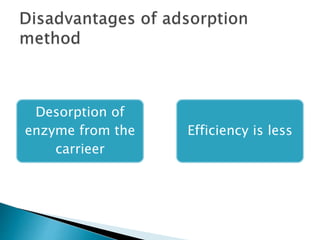
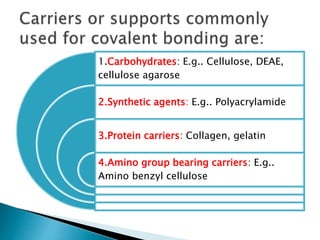
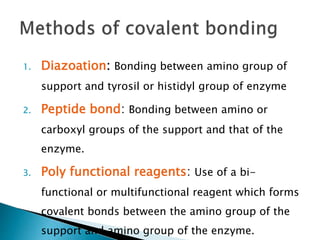
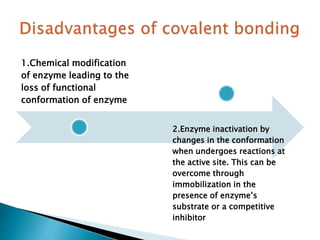
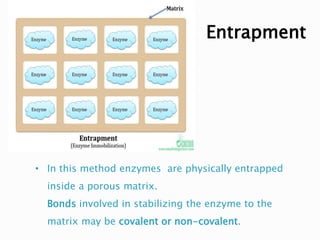
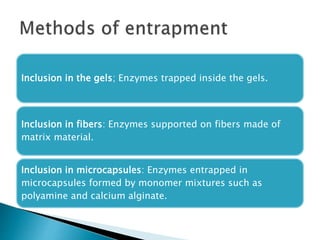
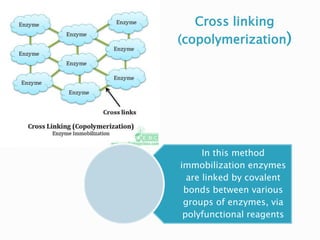
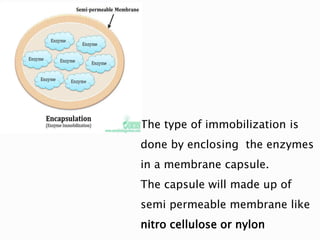
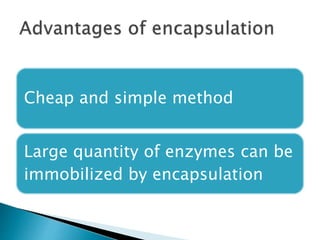
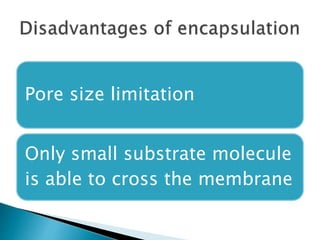
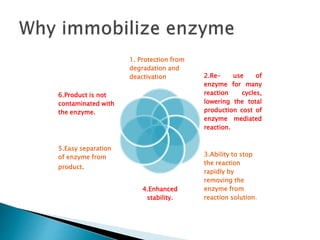
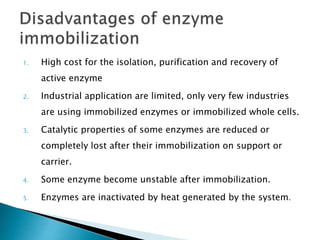
The document discusses immobilization technology for enzymes, detailing methods, supports, advantages, disadvantages, and applications. Key immobilization methods include adsorption, covalent bonding, entrapment, cross-linking, and encapsulation, each with unique characteristics and usage scenarios. Enzyme immobilization is utilized across various industries, such as pharmaceuticals, food production, and wastewater management, highlighting its significance and diverse applications.