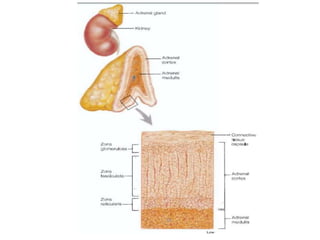
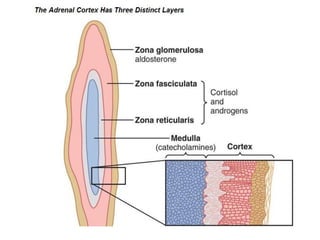
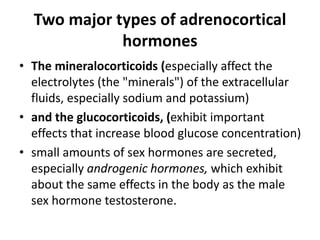
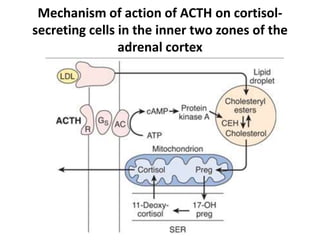
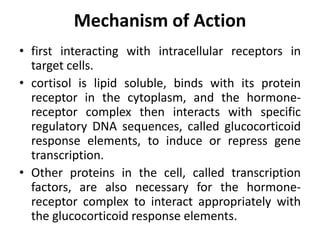
The document discusses adrenocortical hormones and their functions. It notes that the adrenal cortex secretes corticosteroids including cortisol and aldosterone. Cortisol affects carbohydrate, protein, and fat metabolism. It stimulates gluconeogenesis and mobilizes fats and proteins. Cortisol also has anti-inflammatory effects and plays an important role in the body's response to stress. Cortisol secretion is regulated by ACTH from the pituitary gland which stimulates production through the cAMP pathway.





































































![• Cushing's disease refers only to hypercortisolism secondary
to excess production of ACTH from a corticotroph pituitary
adenoma (secondary hypercortisolism/hypercorticism) or
due to excess production of hypothalamus CRH
(Corticotropin releasing hormone) (tertiary
hypercortisolism/hypercorticism). This causes the blood
ACTH levels to be elevated along with cortisol from the
adrenal gland. The ACTH levels remain high because the
tumor is unresponsive to negative feedback from high
cortisol levels.
• Cushing's disease is not to be confused with ectopic
Cushing syndrome[21] (ectopic ACTH syndrome), which is
often seen in paraneoplastic syndrome.](https://image.slidesharecdn.com/adrenocorticalhormones-170505150007/85/Adrenocortical-hormones-76-320.jpg)






![Cushing's syndrome
• describes the signs and
symptoms associated with
prolonged exposure to
inappropriately high levels of
the hormone cortisol. This can
be caused by
taking glucocorticoid drugs, or
diseases that result in excess
cortisol, adrenocorticotropic
hormone (ACTH), or CRH
levels.[1]
Cushing's disease
• refers to a pituitary-
dependent cause of Cushing's
syndrome: a tumor
(adenoma) in the pituitary
gland produces large amounts
of ACTH, causing the adrenal
glands to produce elevated
levels of cortisol.
• It is the most common non-
iatrogeniccause of Cushing's
syndrome, responsible for
70% of cases excluding
glucocorticoid related
cases.[2][3]](https://image.slidesharecdn.com/adrenocorticalhormones-170505150007/85/Adrenocortical-hormones-83-320.jpg)









