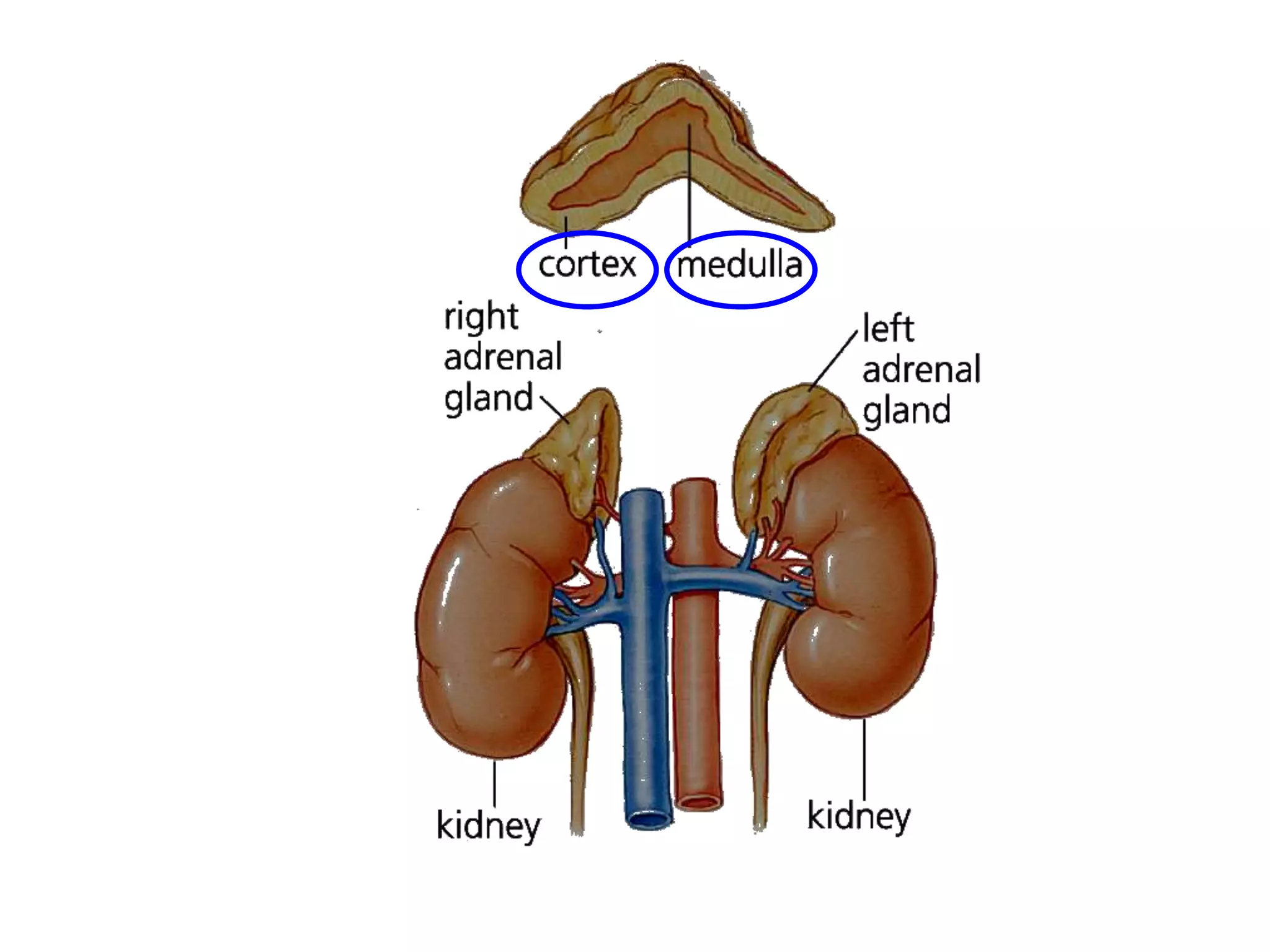
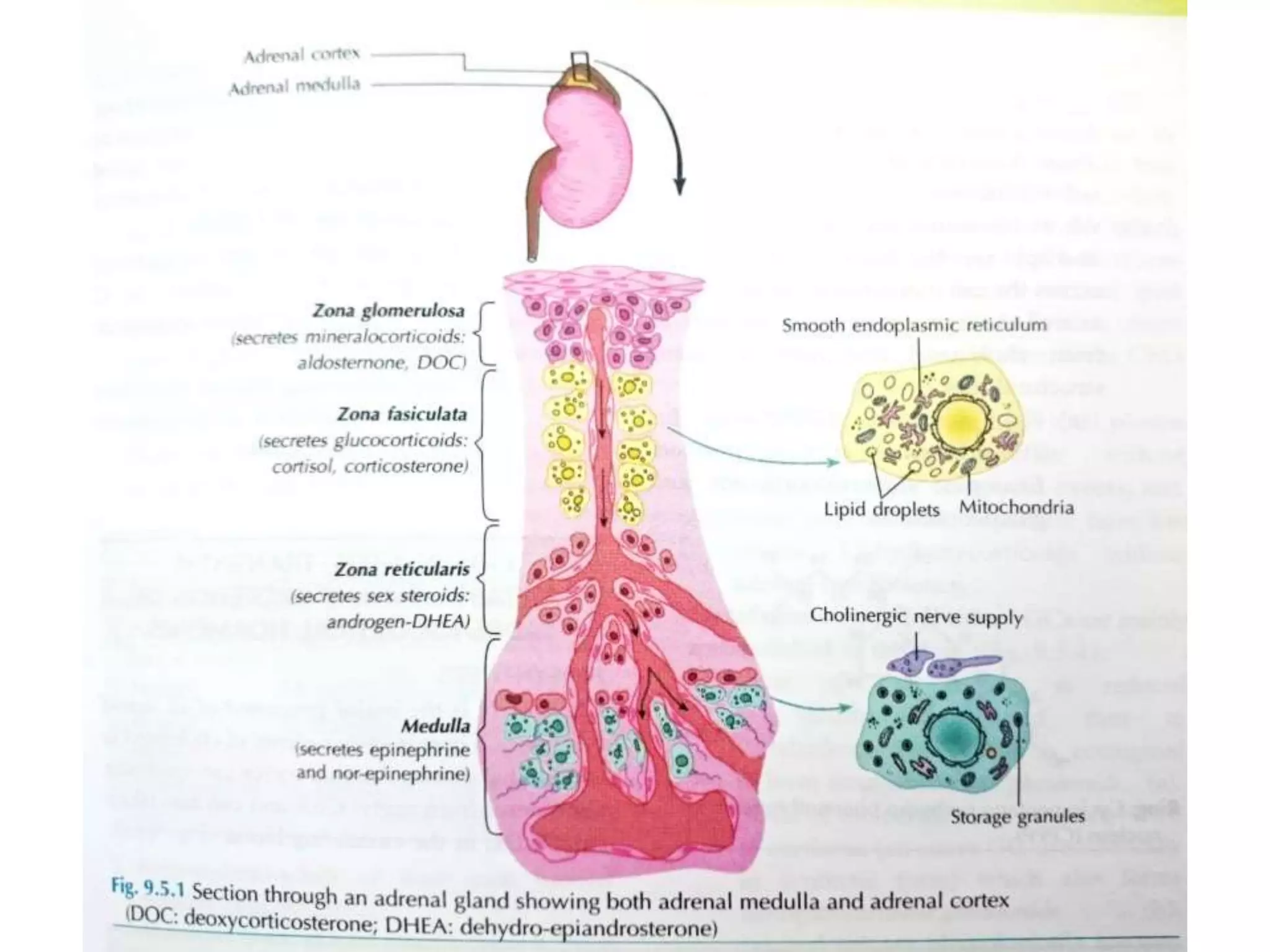
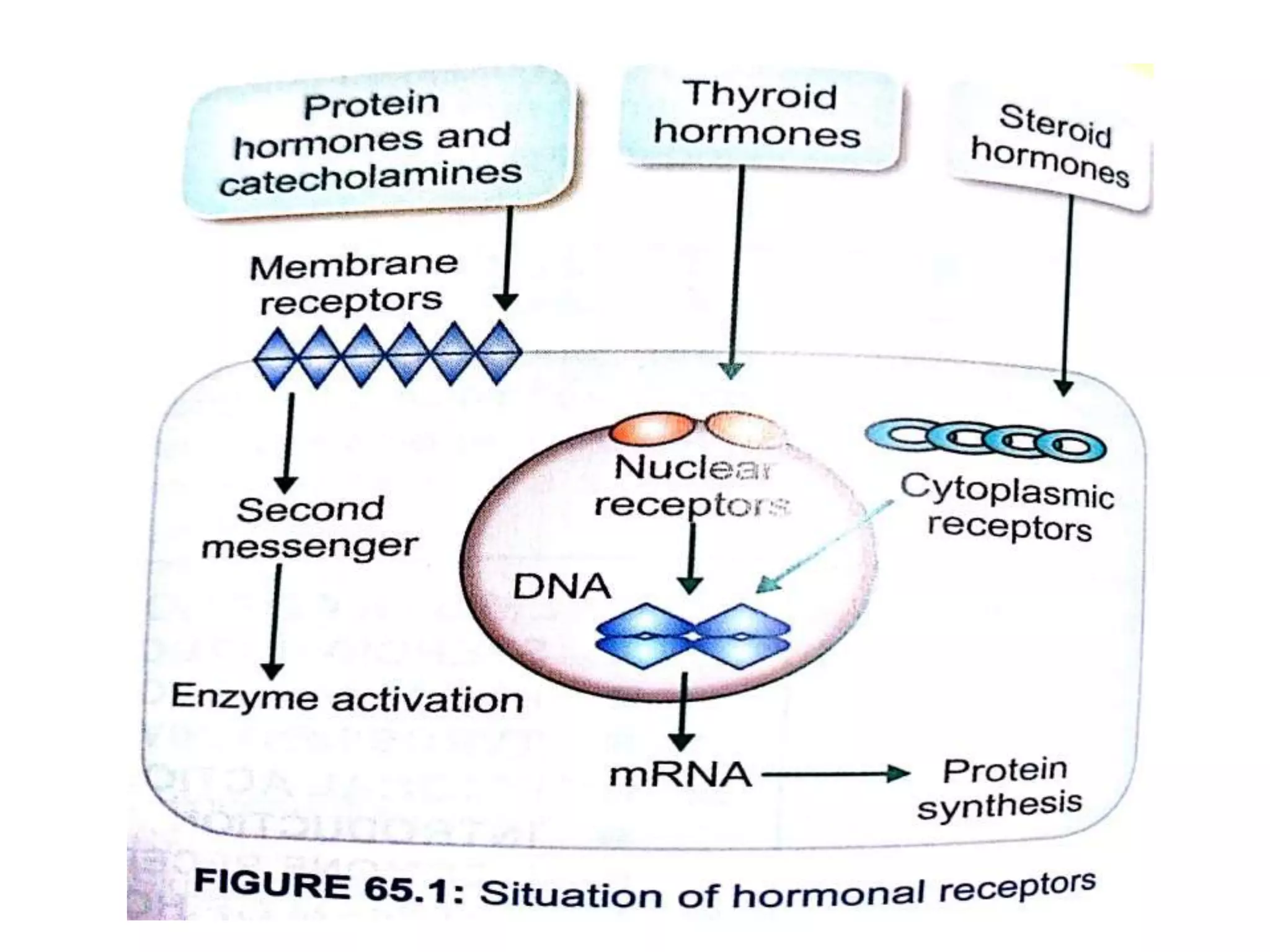
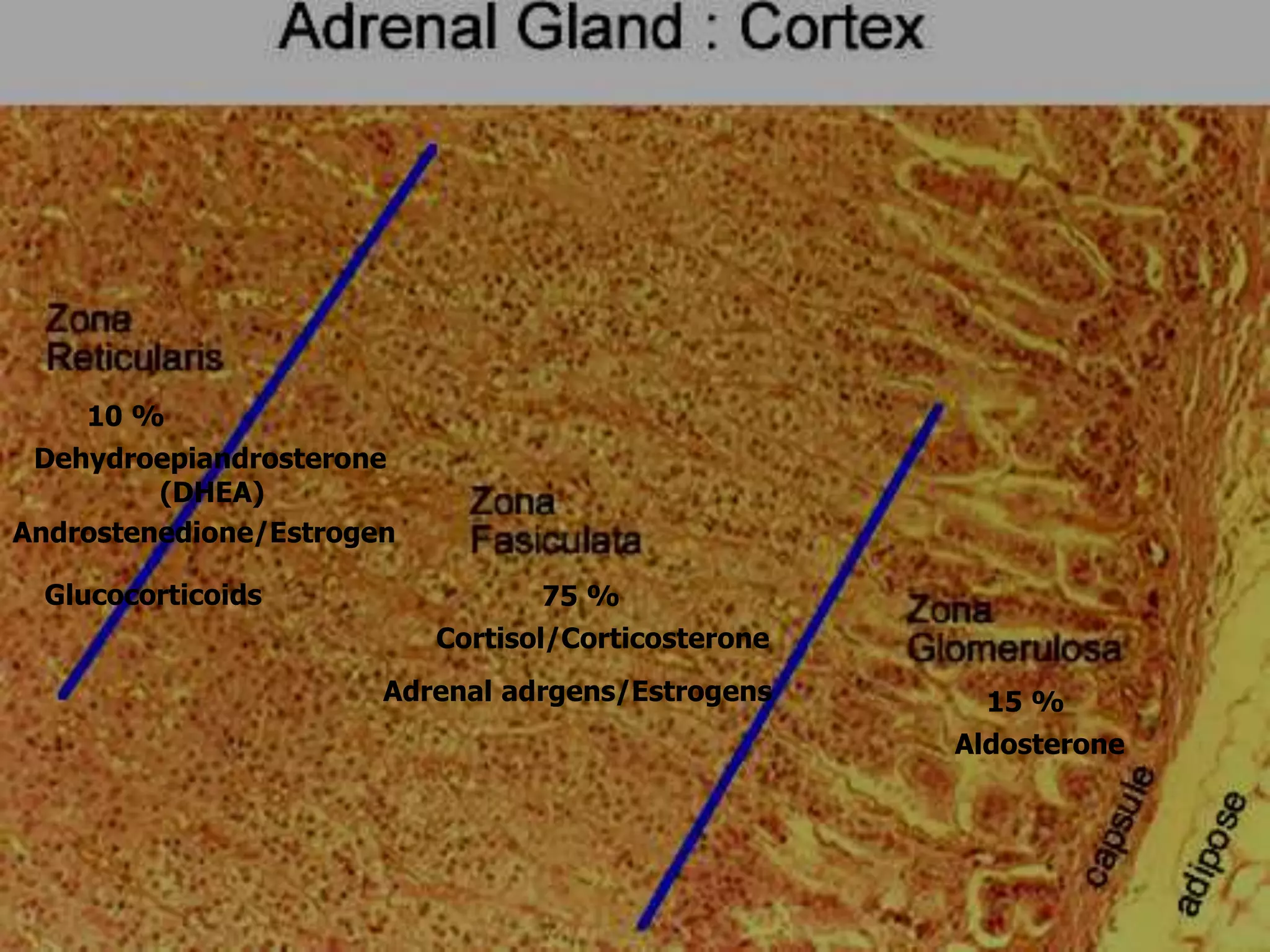
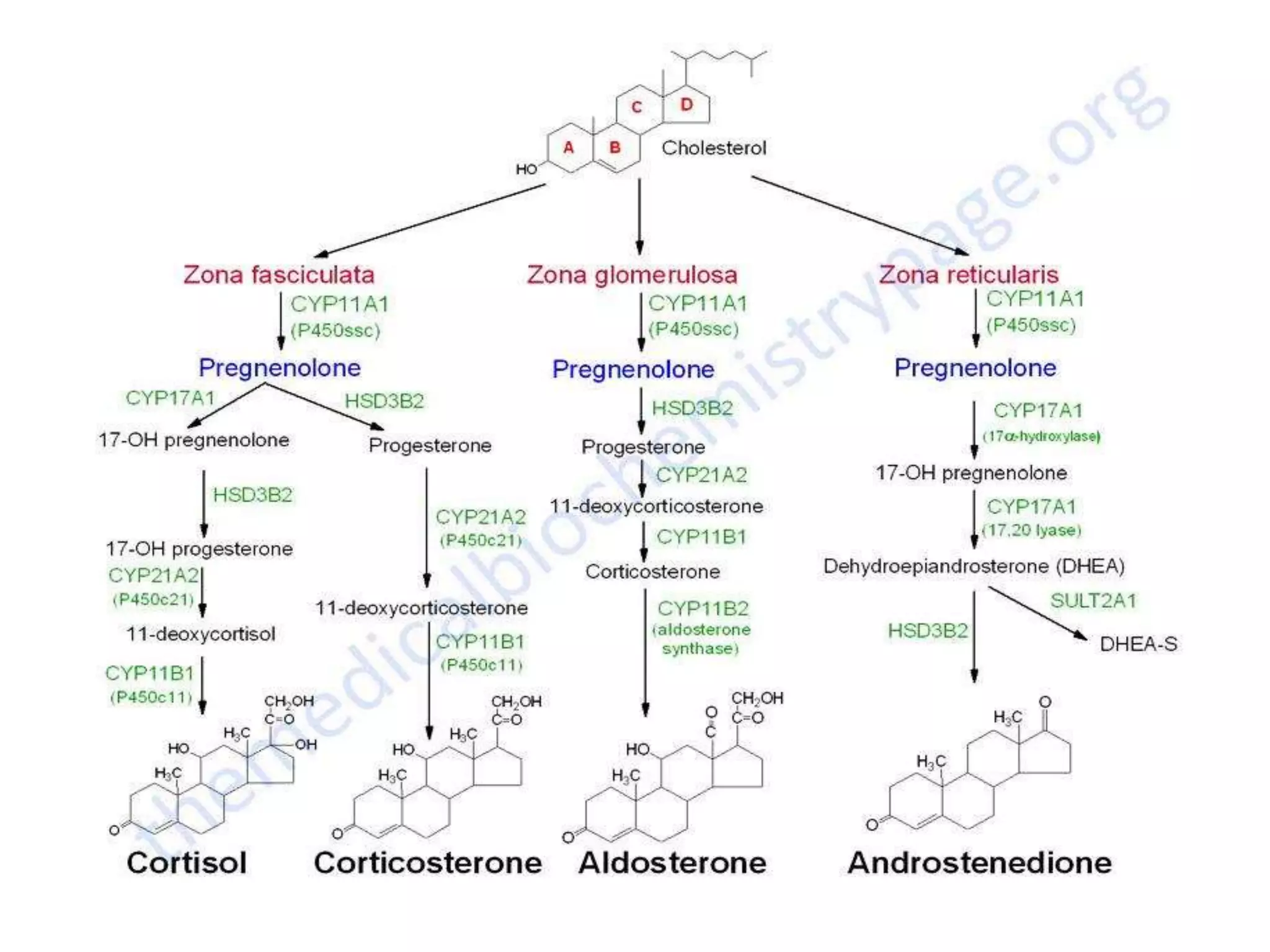
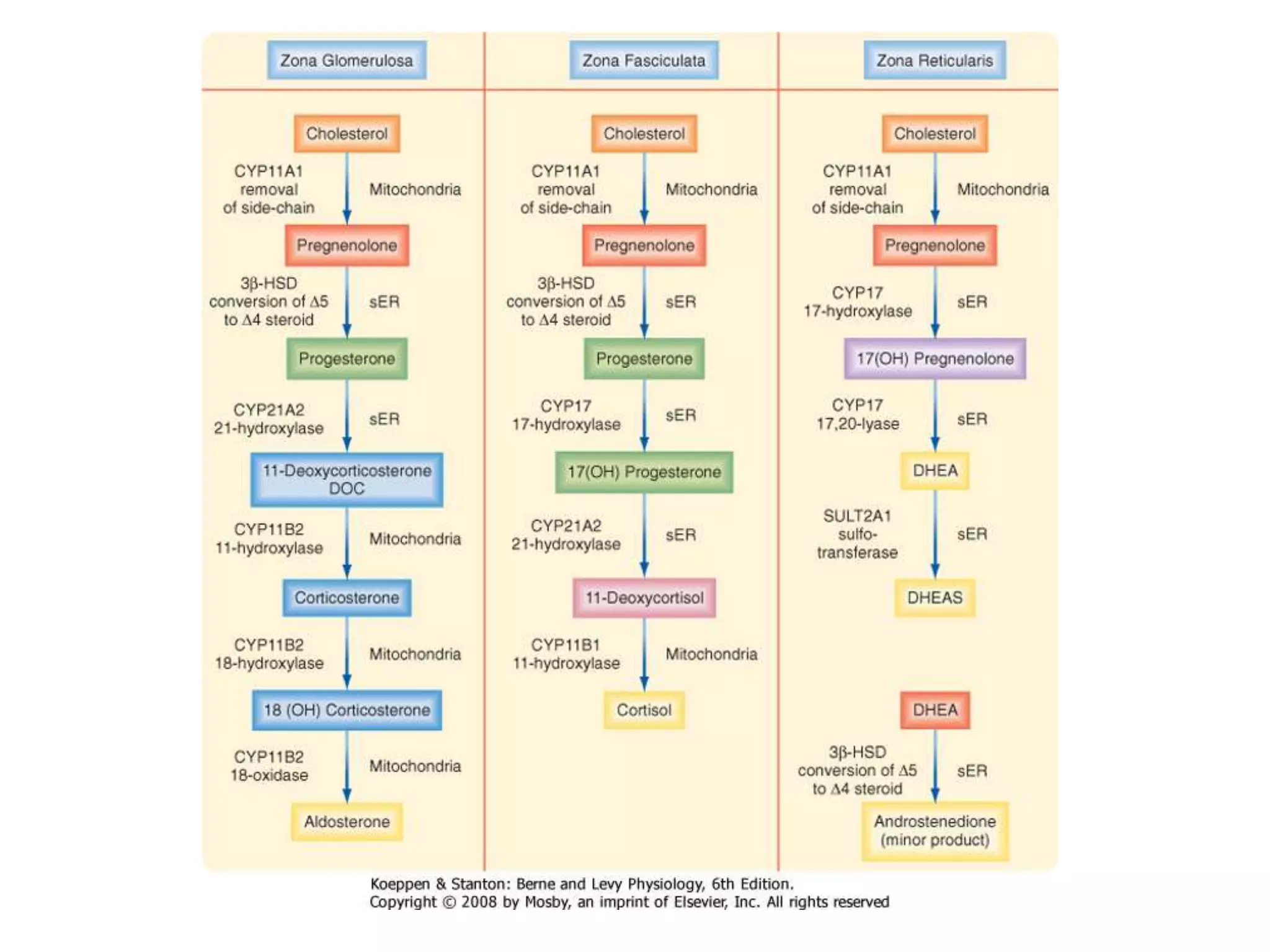
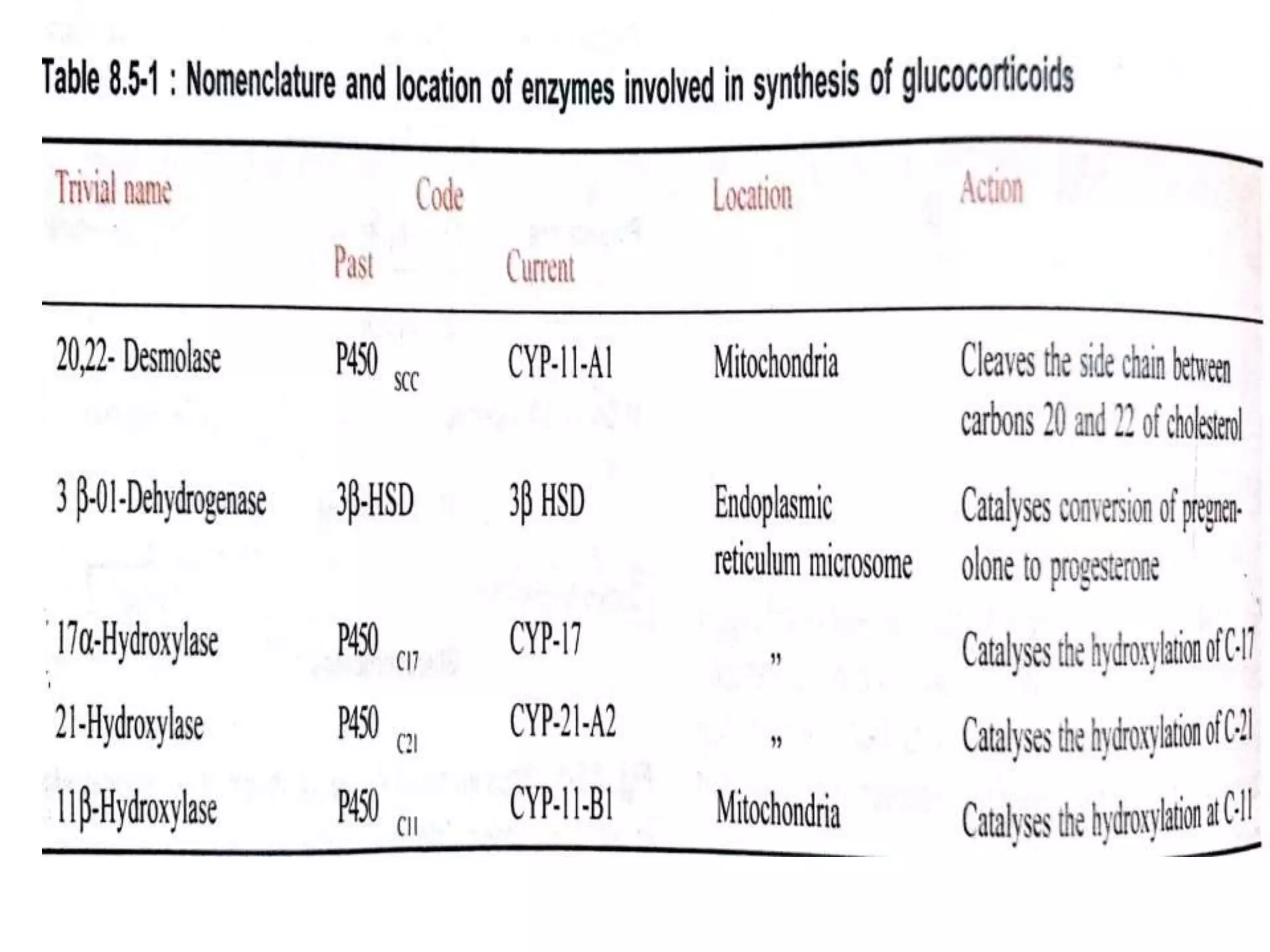
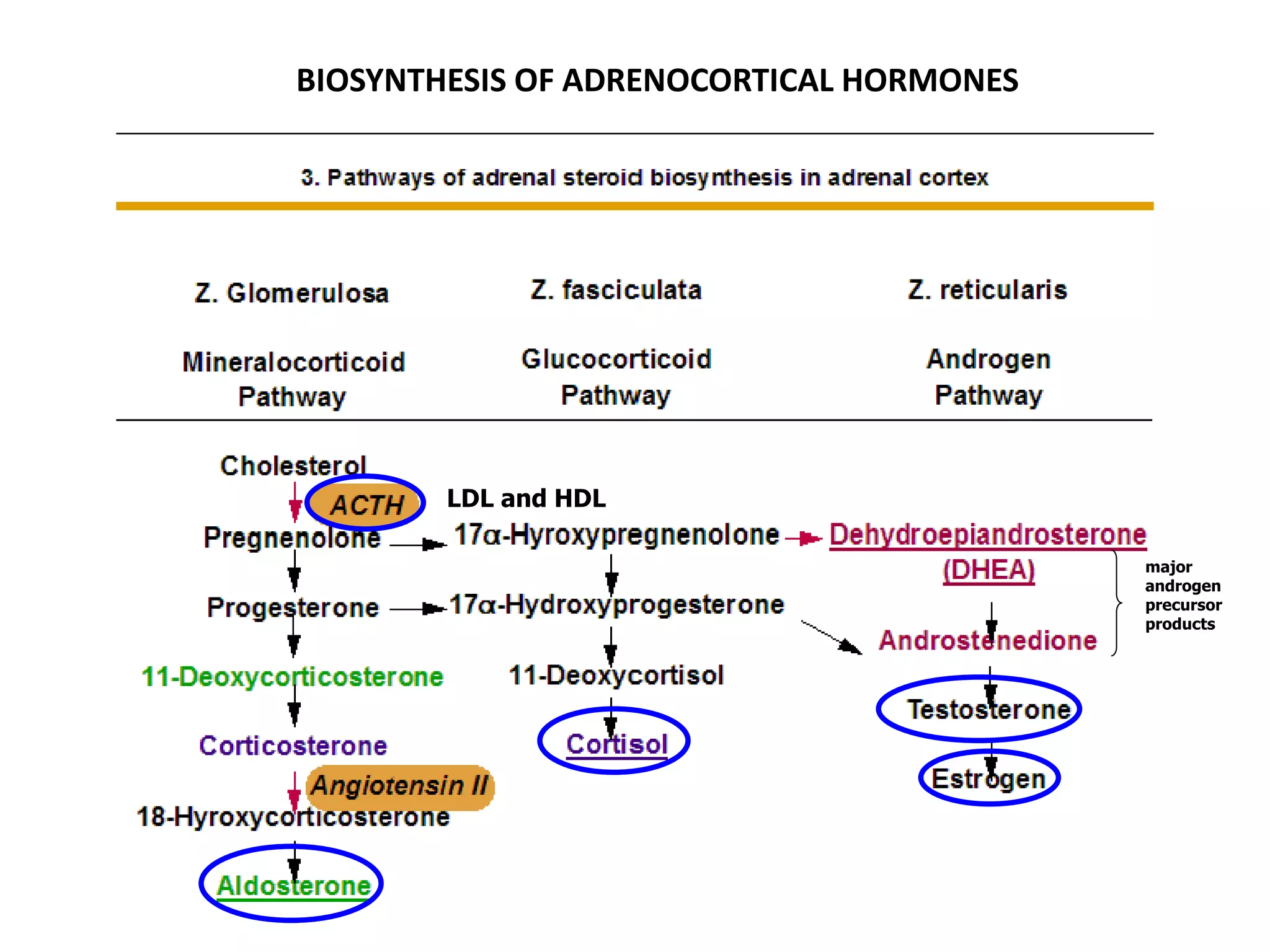
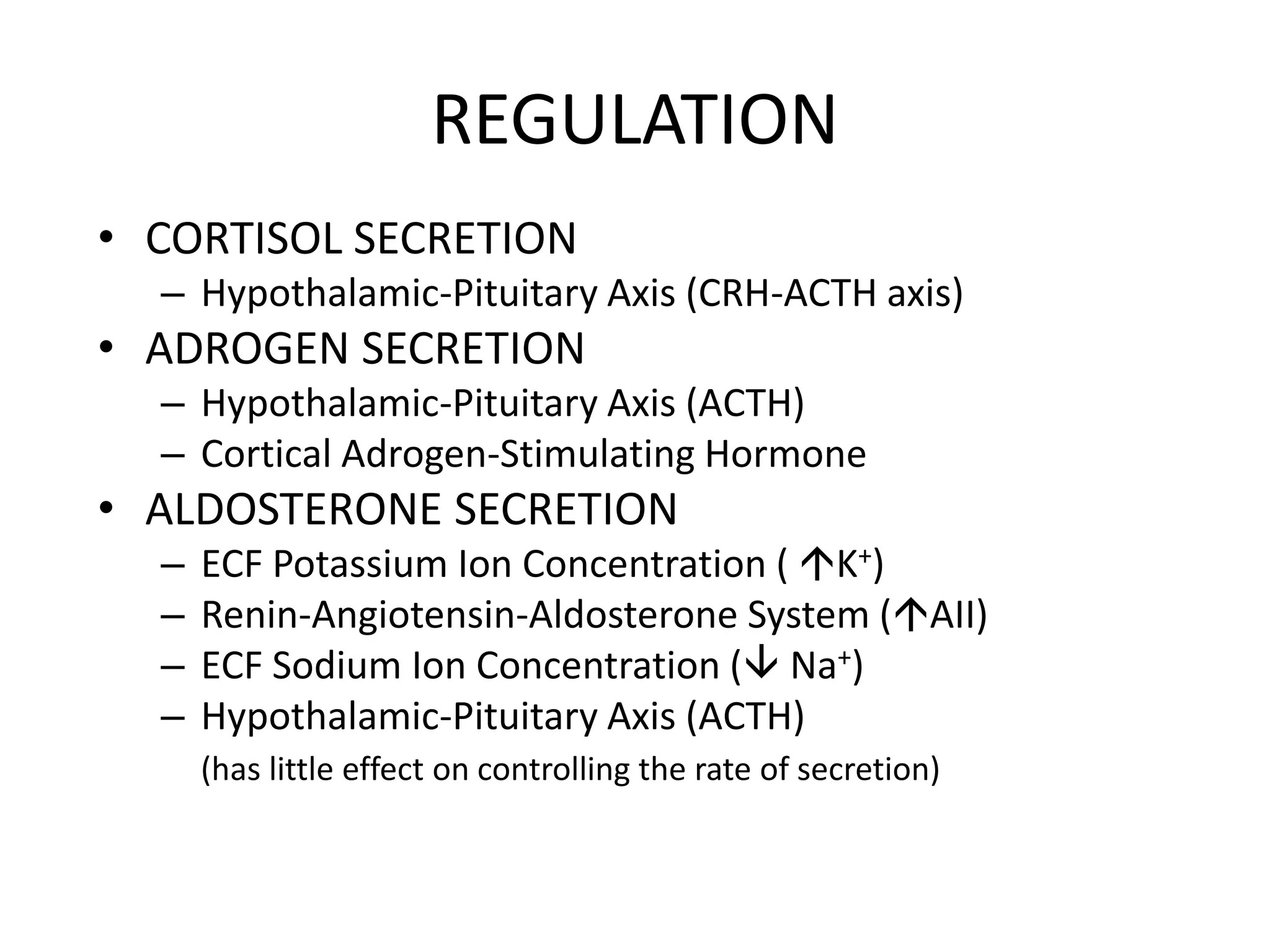
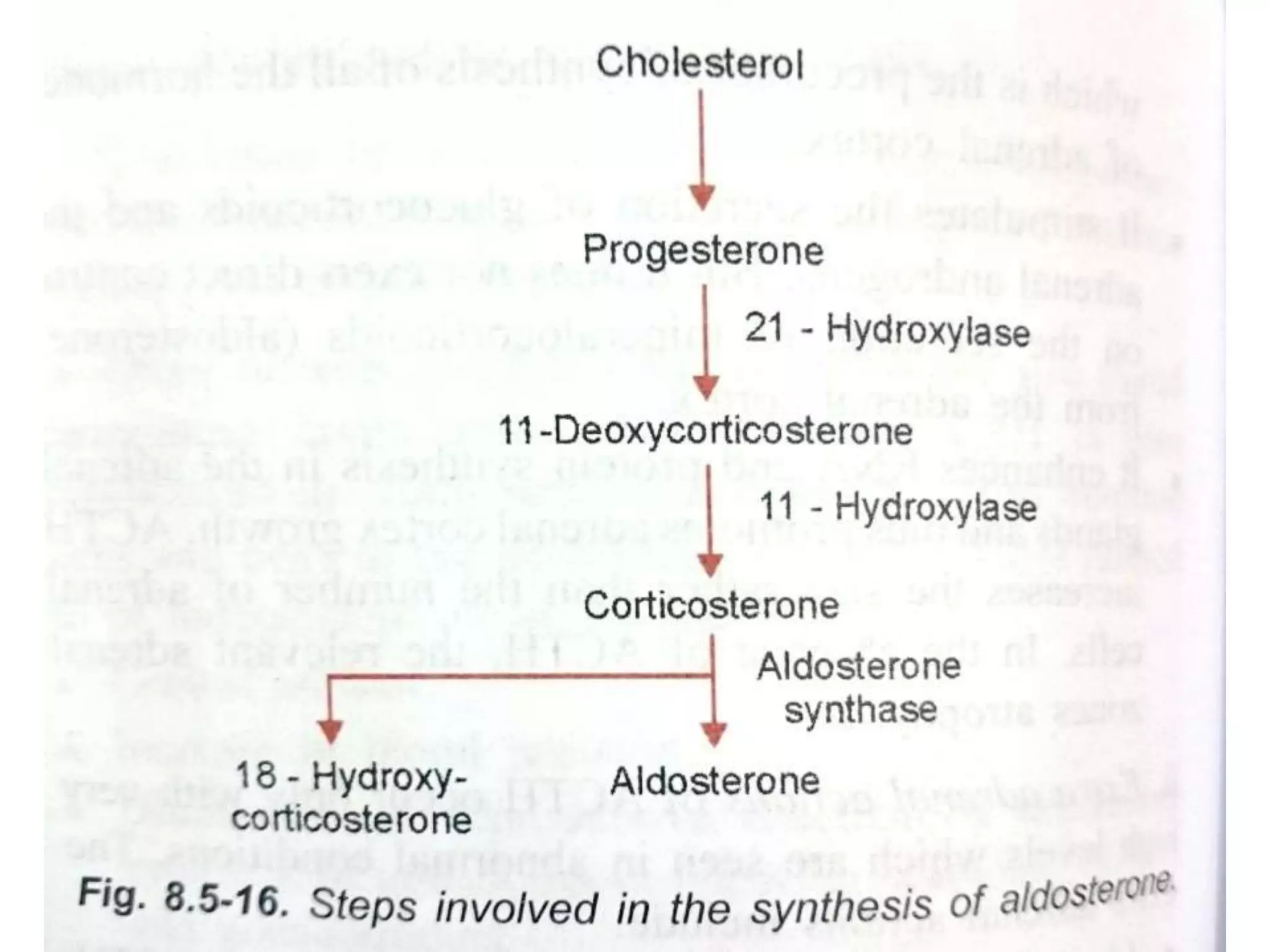
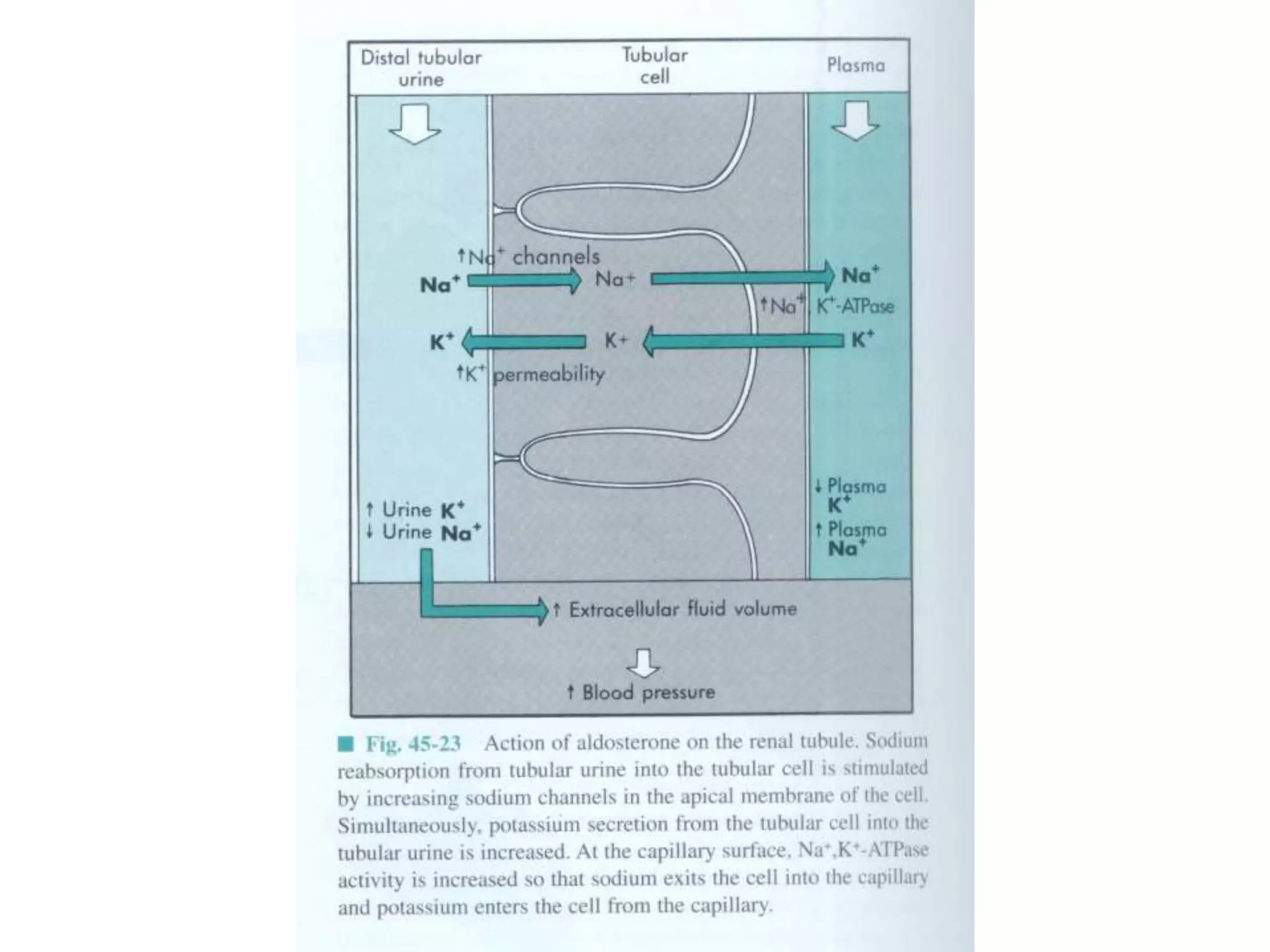
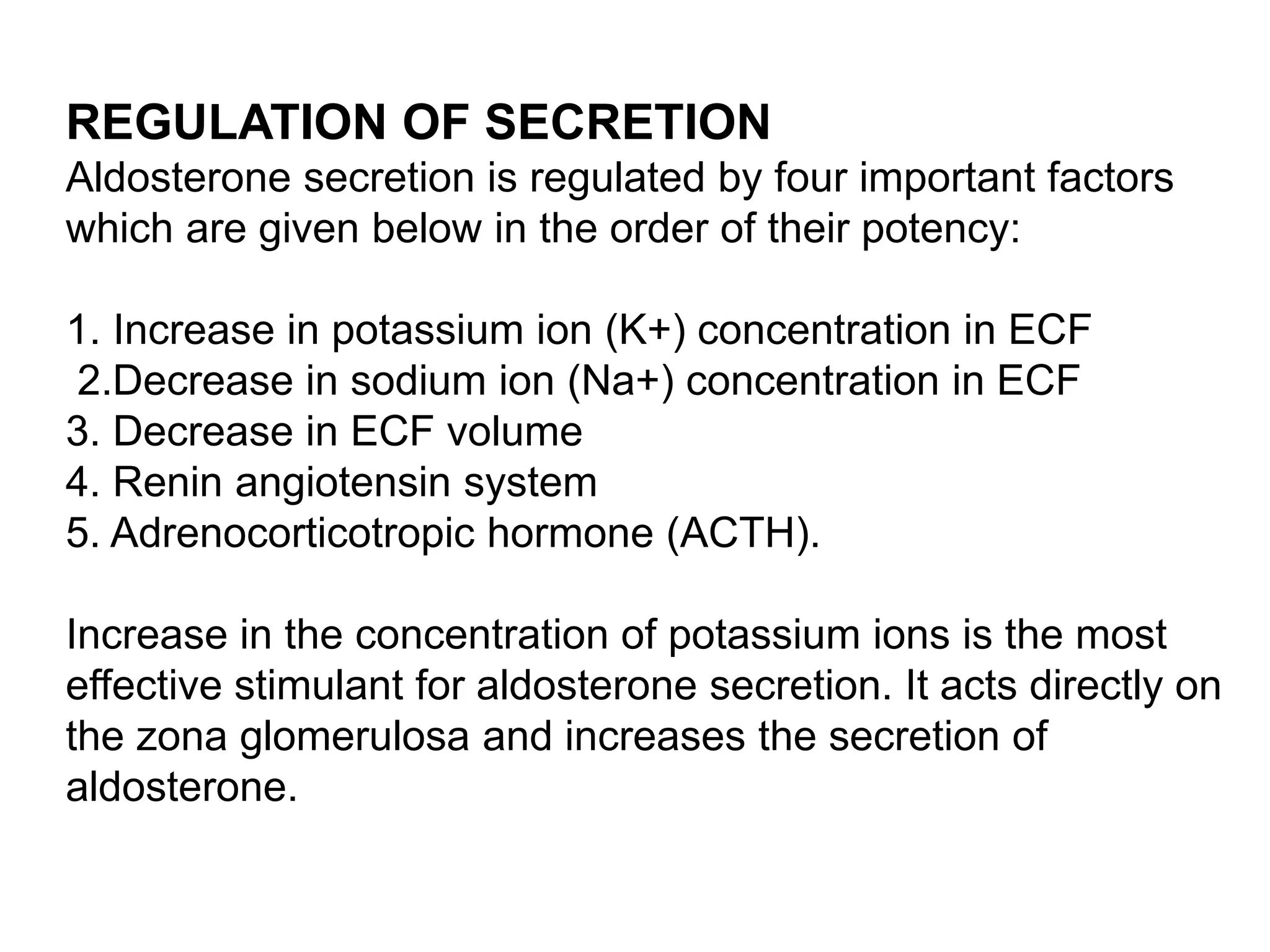
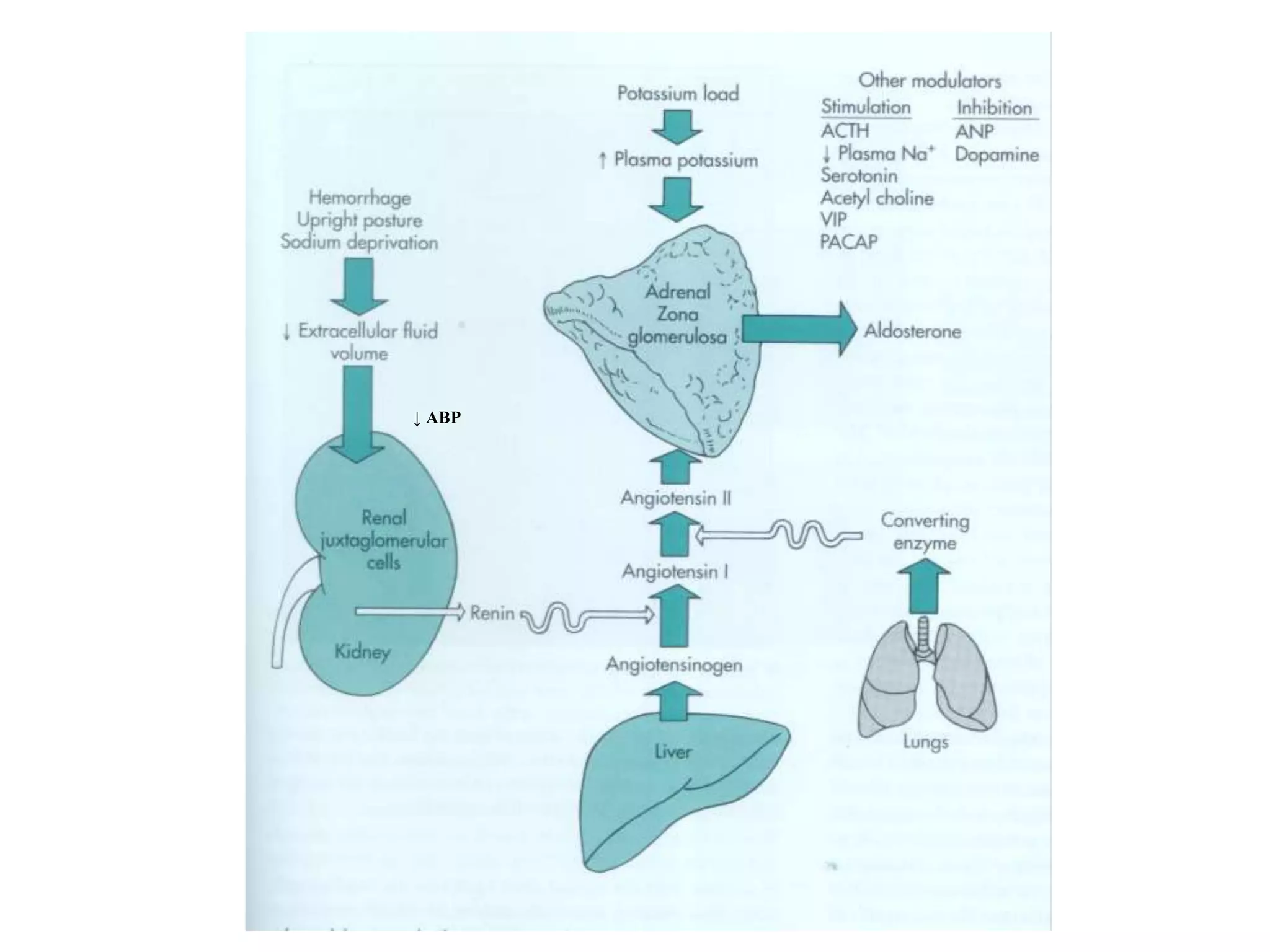
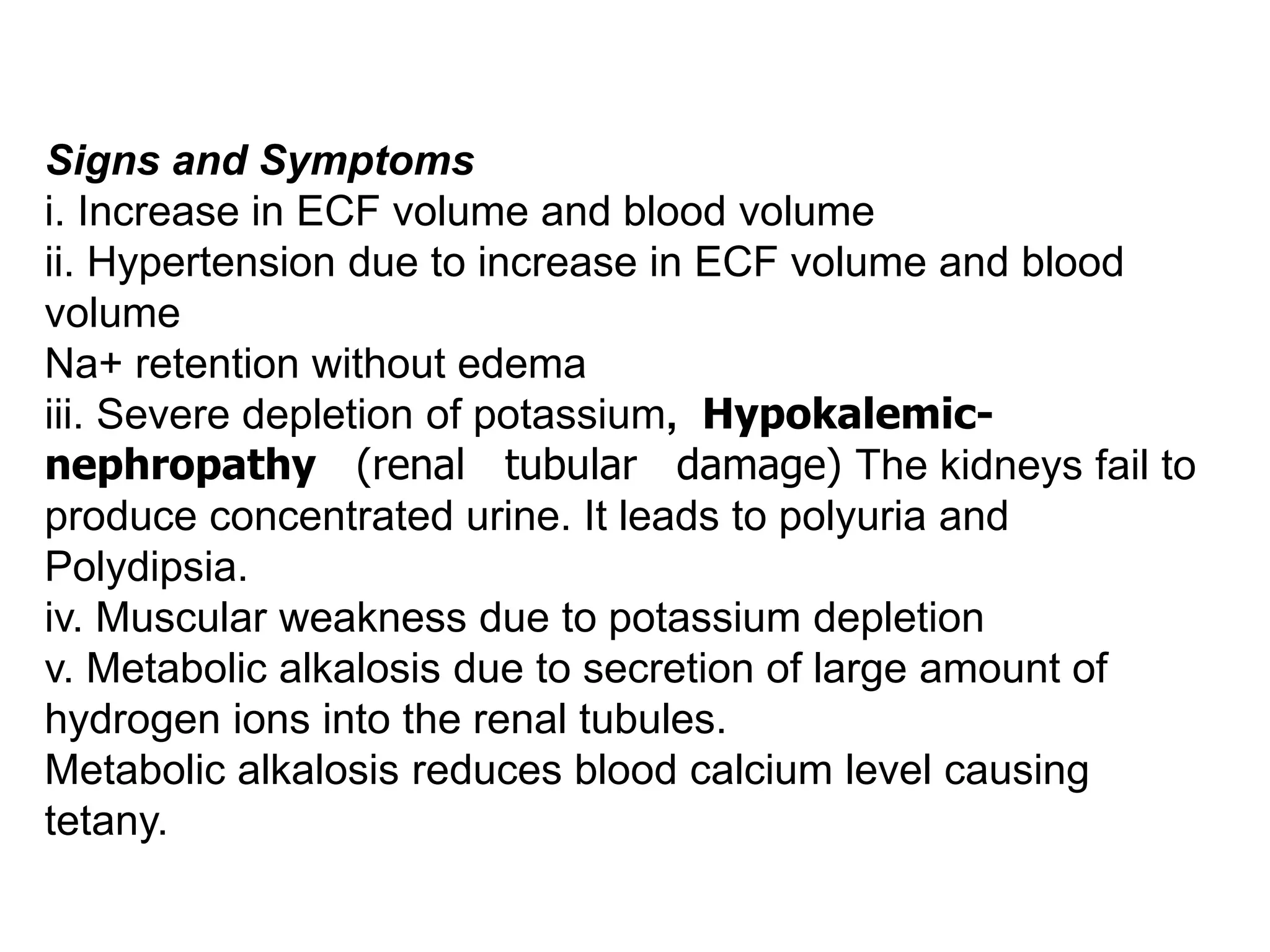
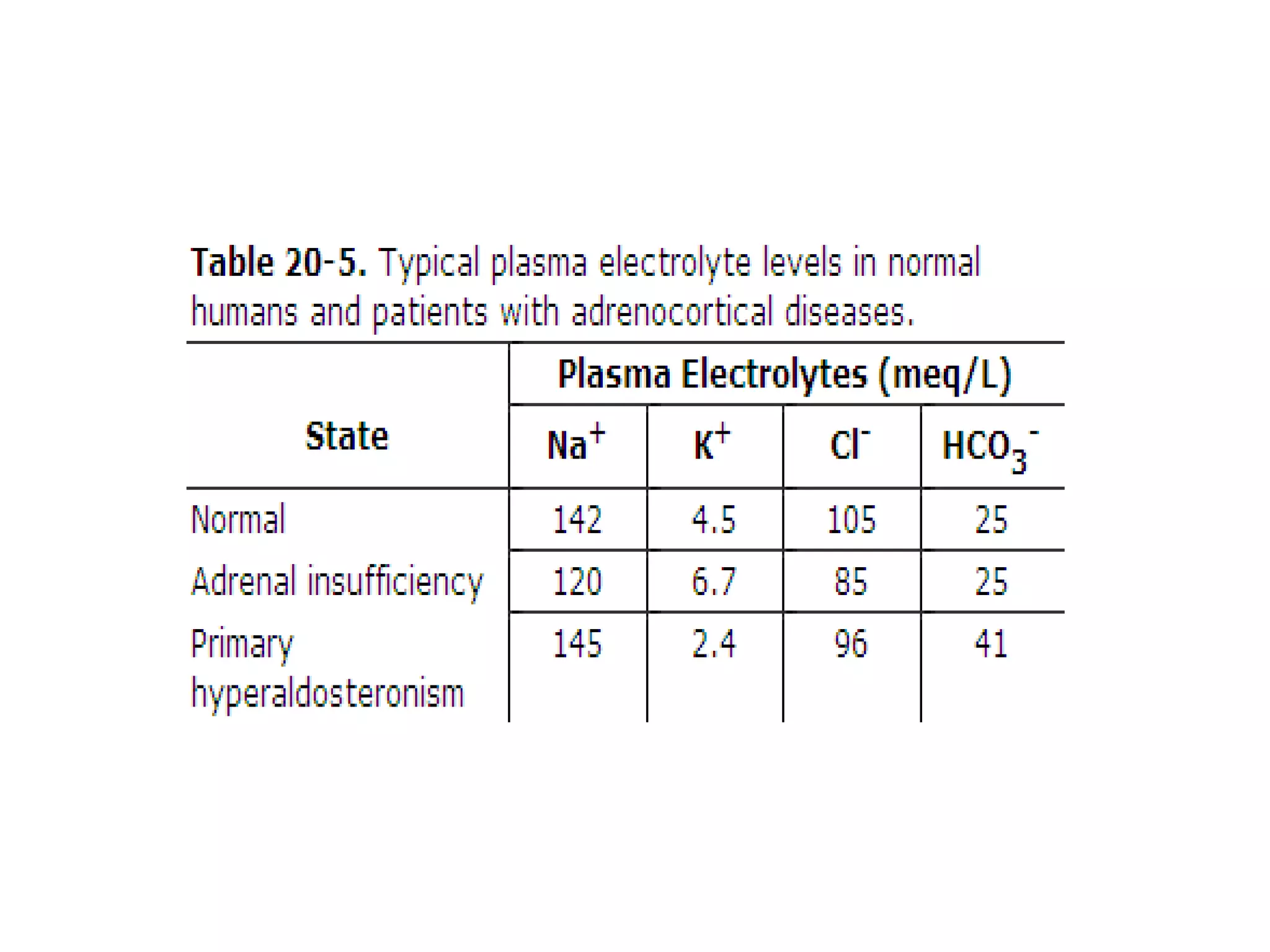
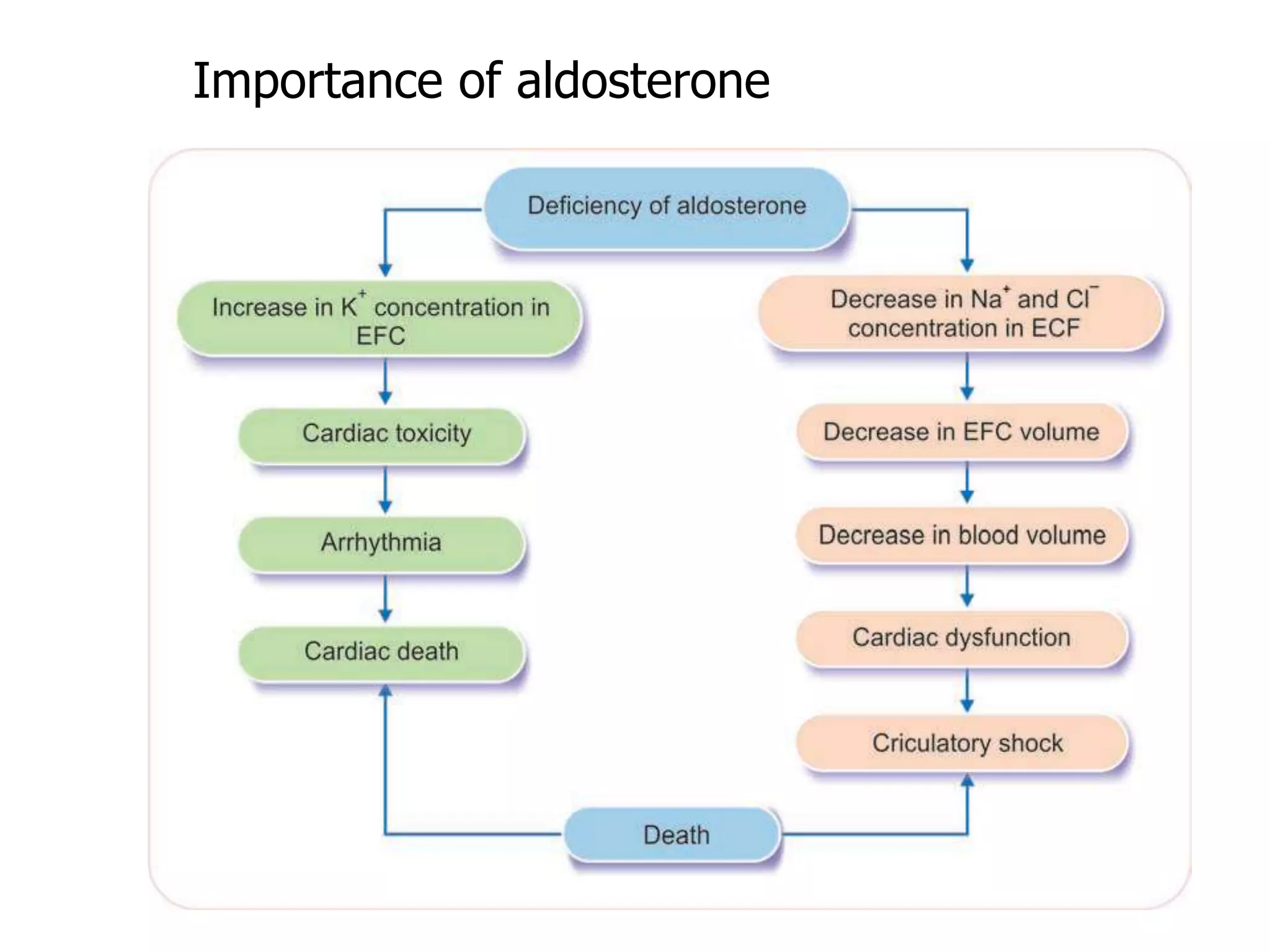
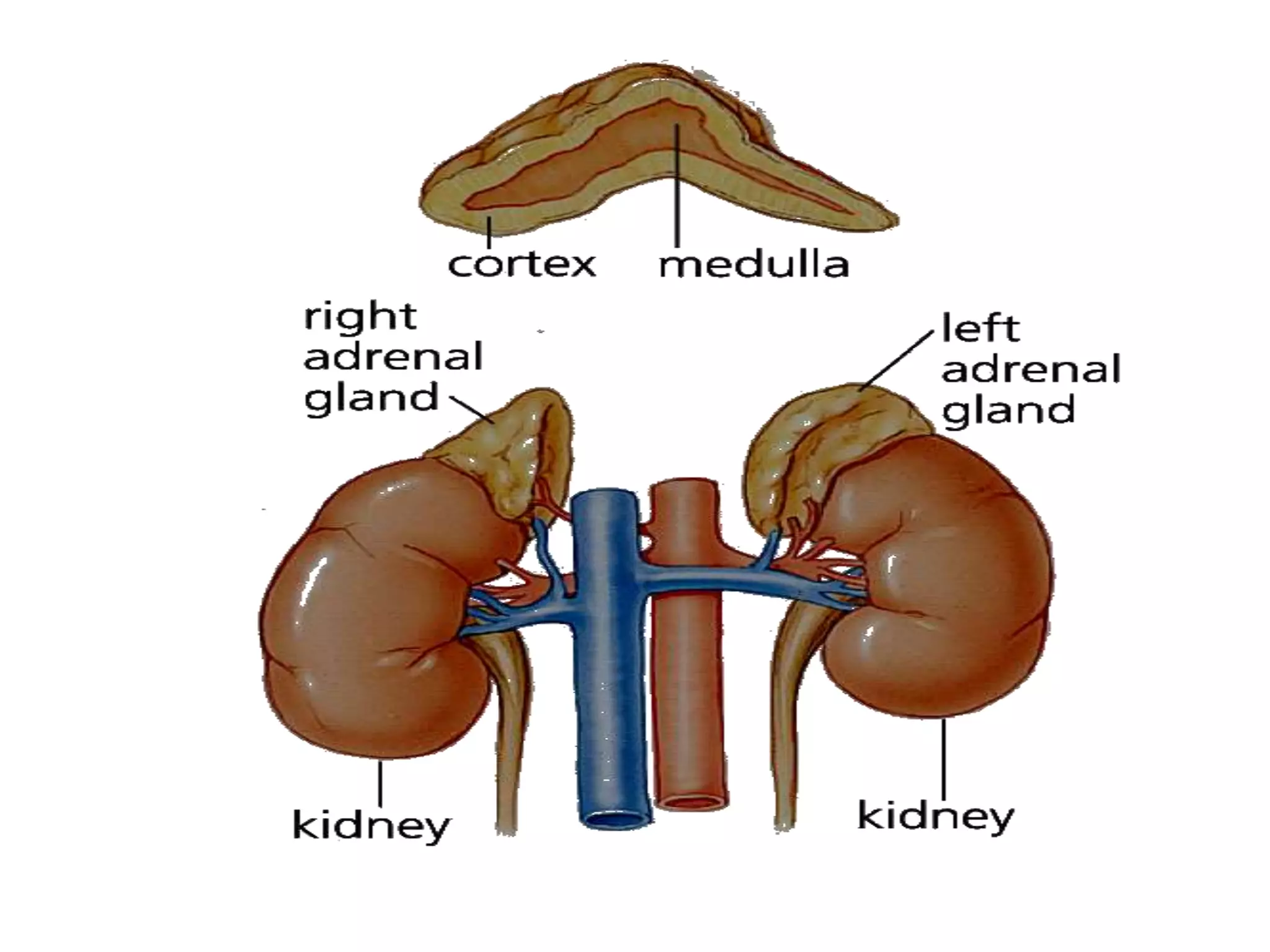
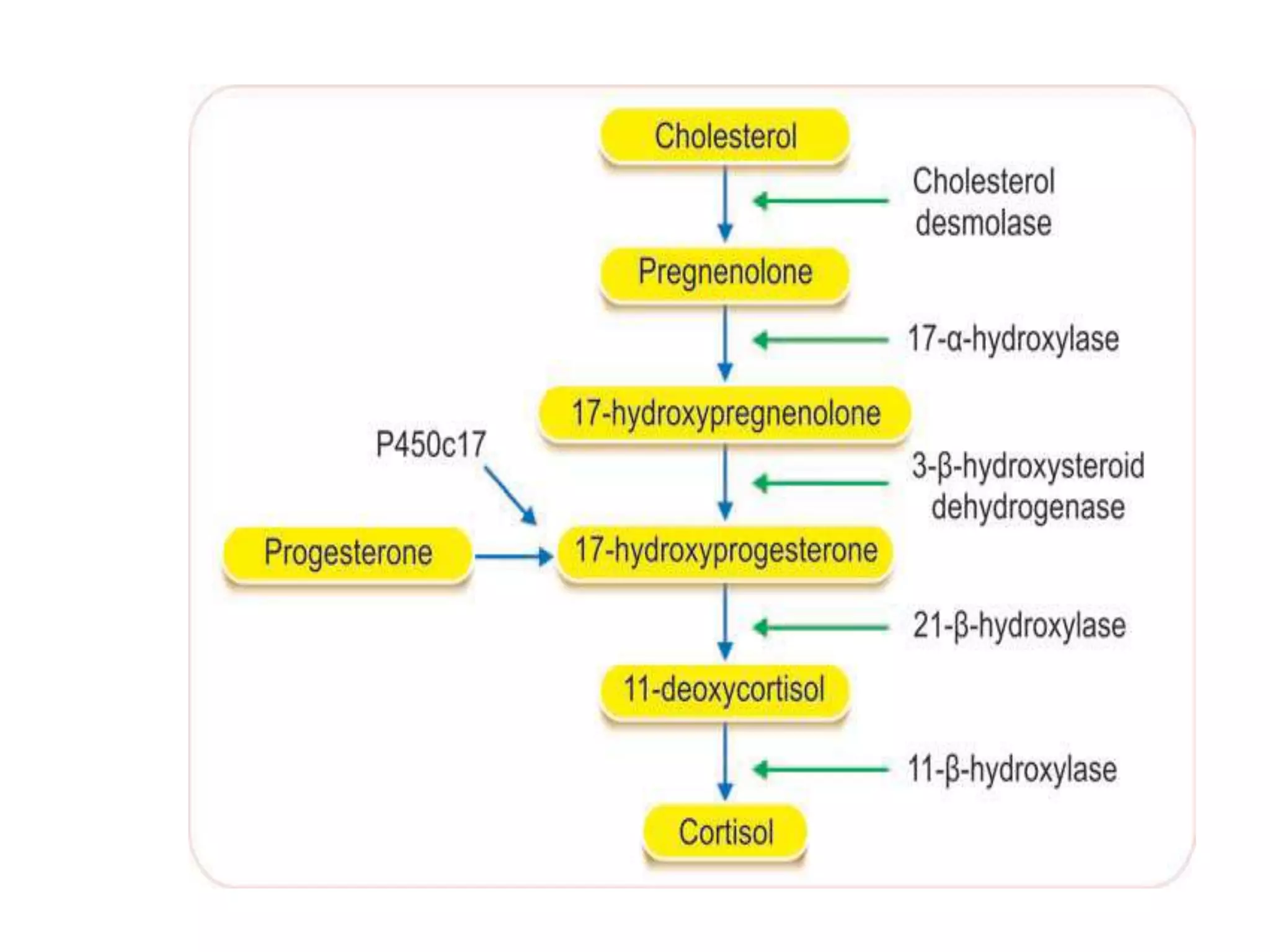
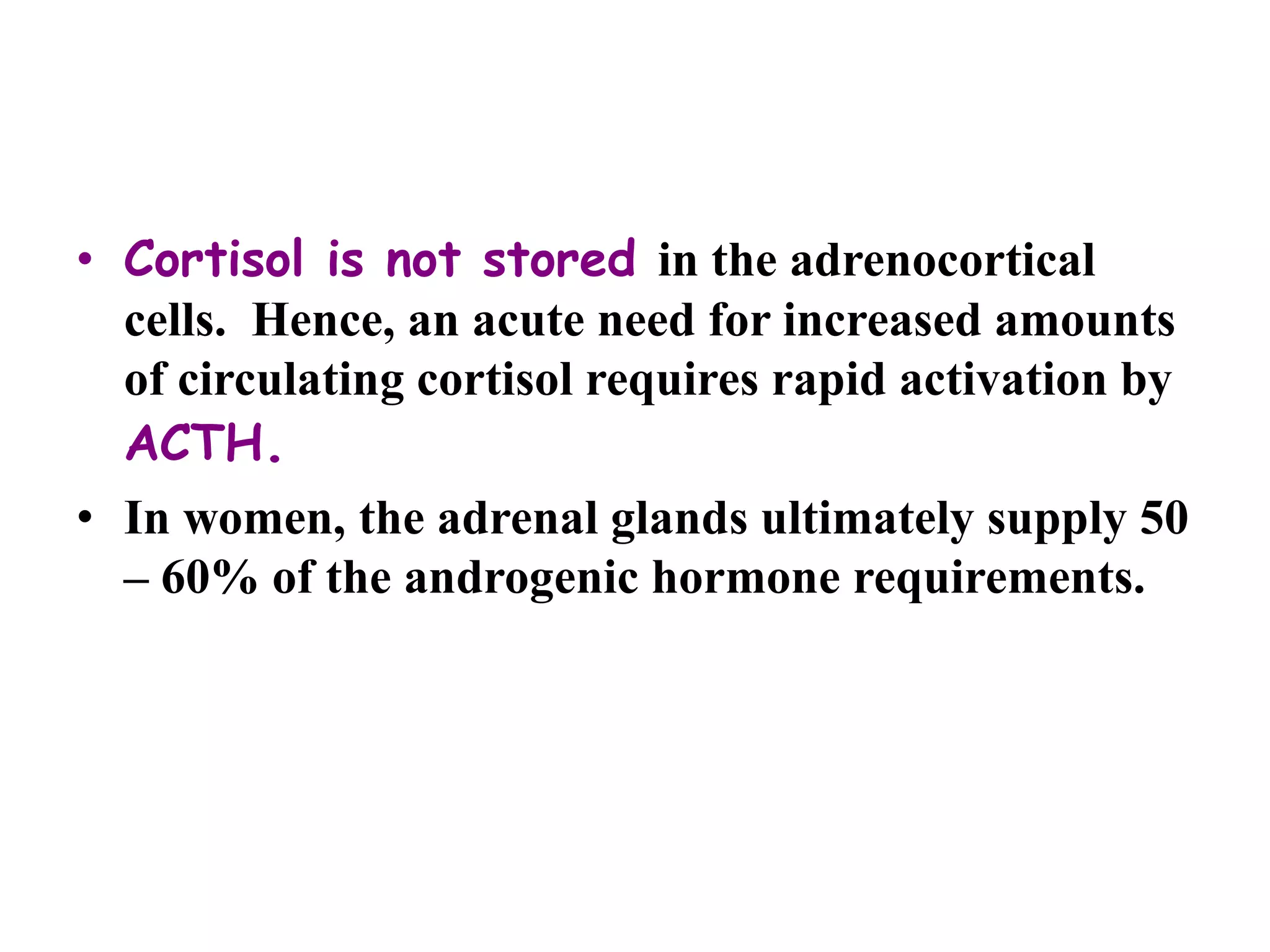
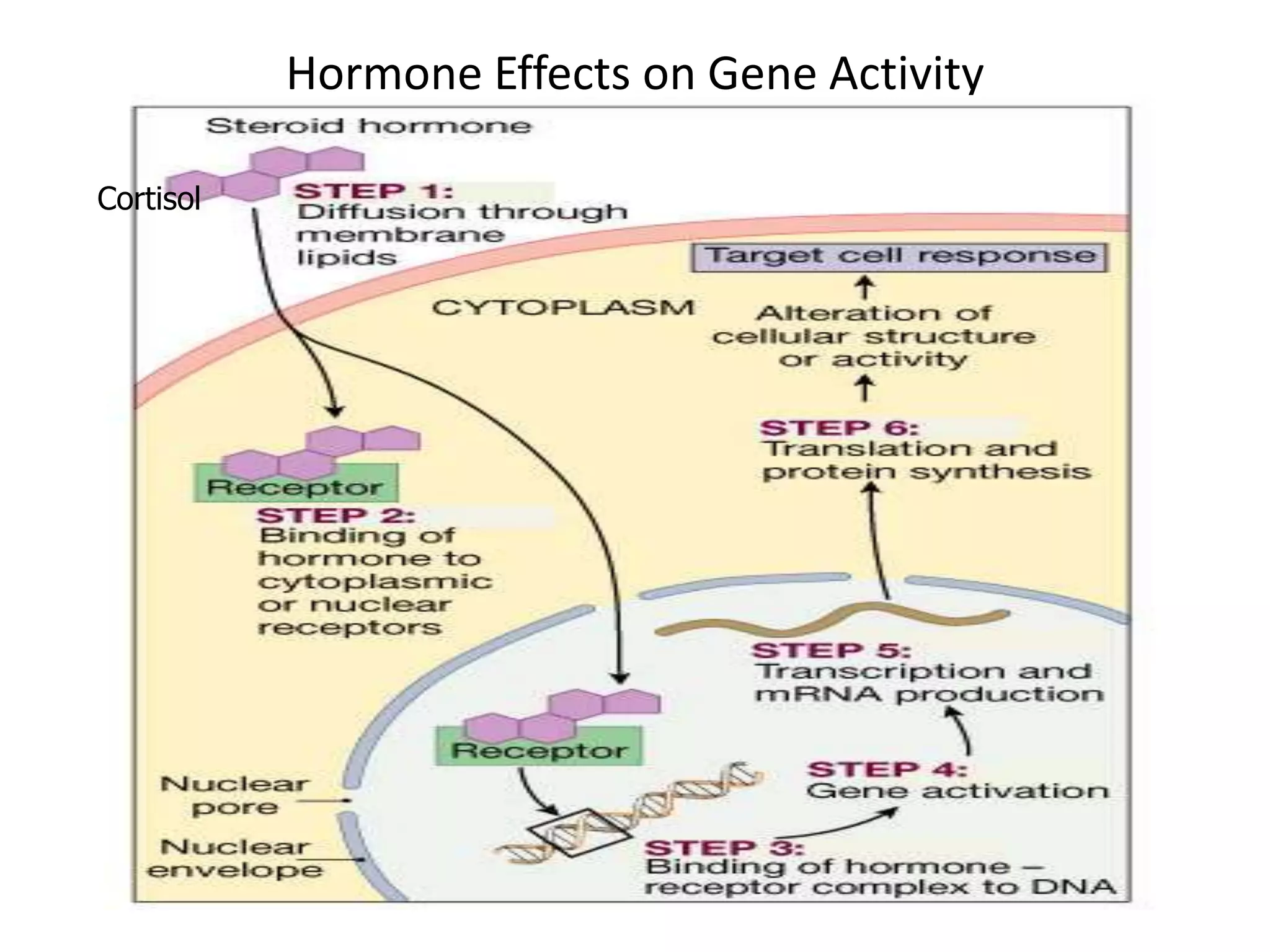
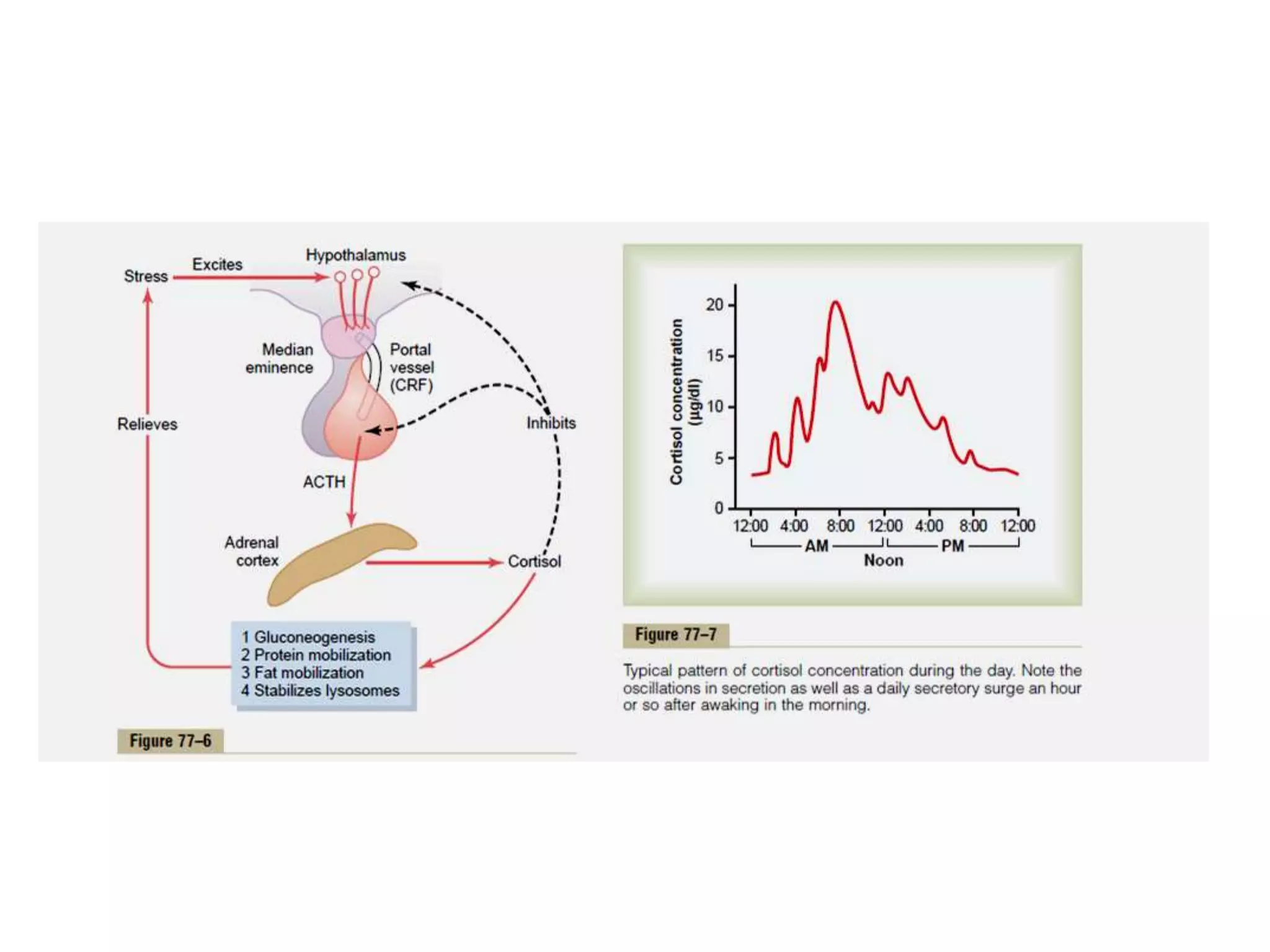
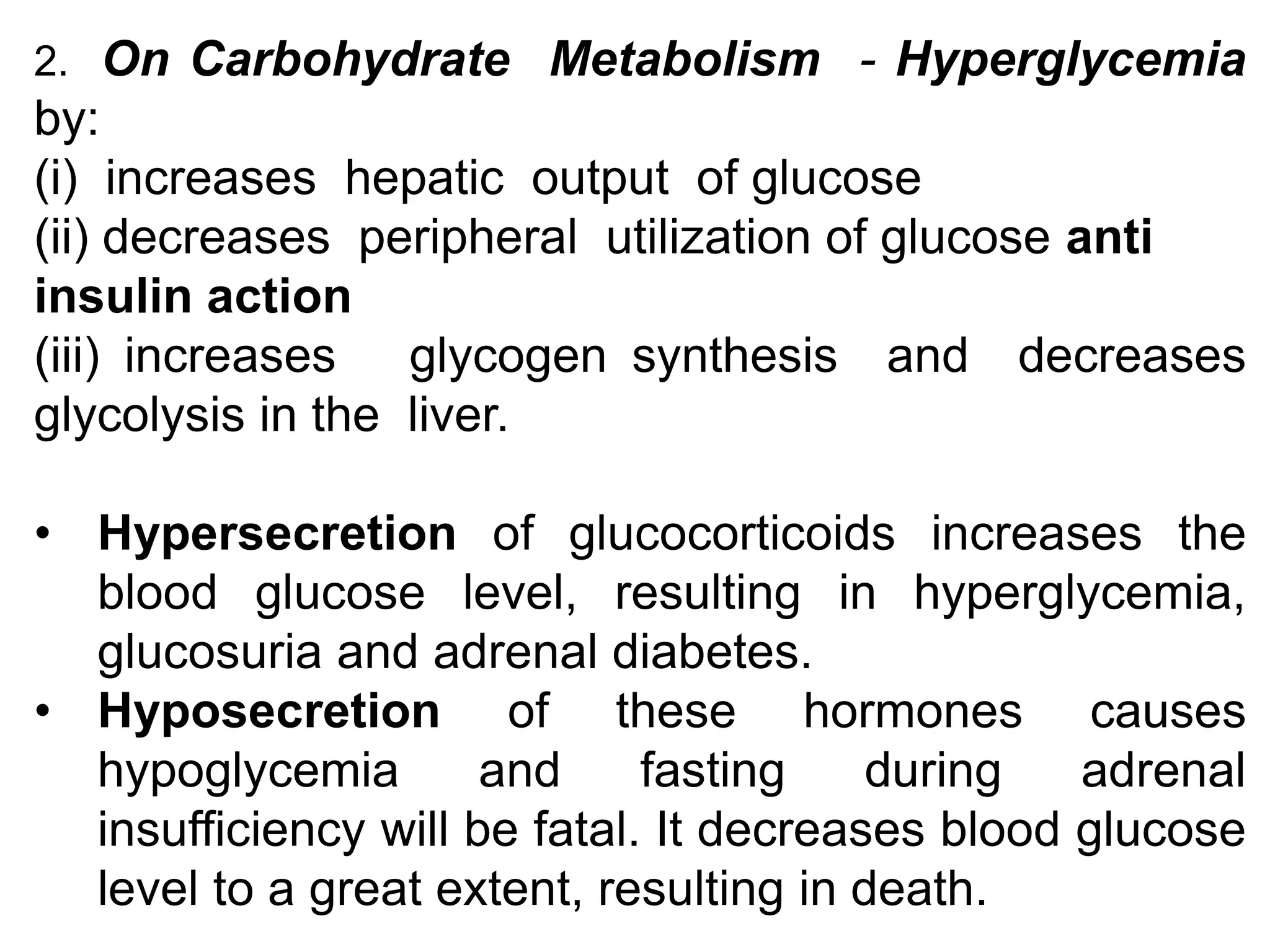
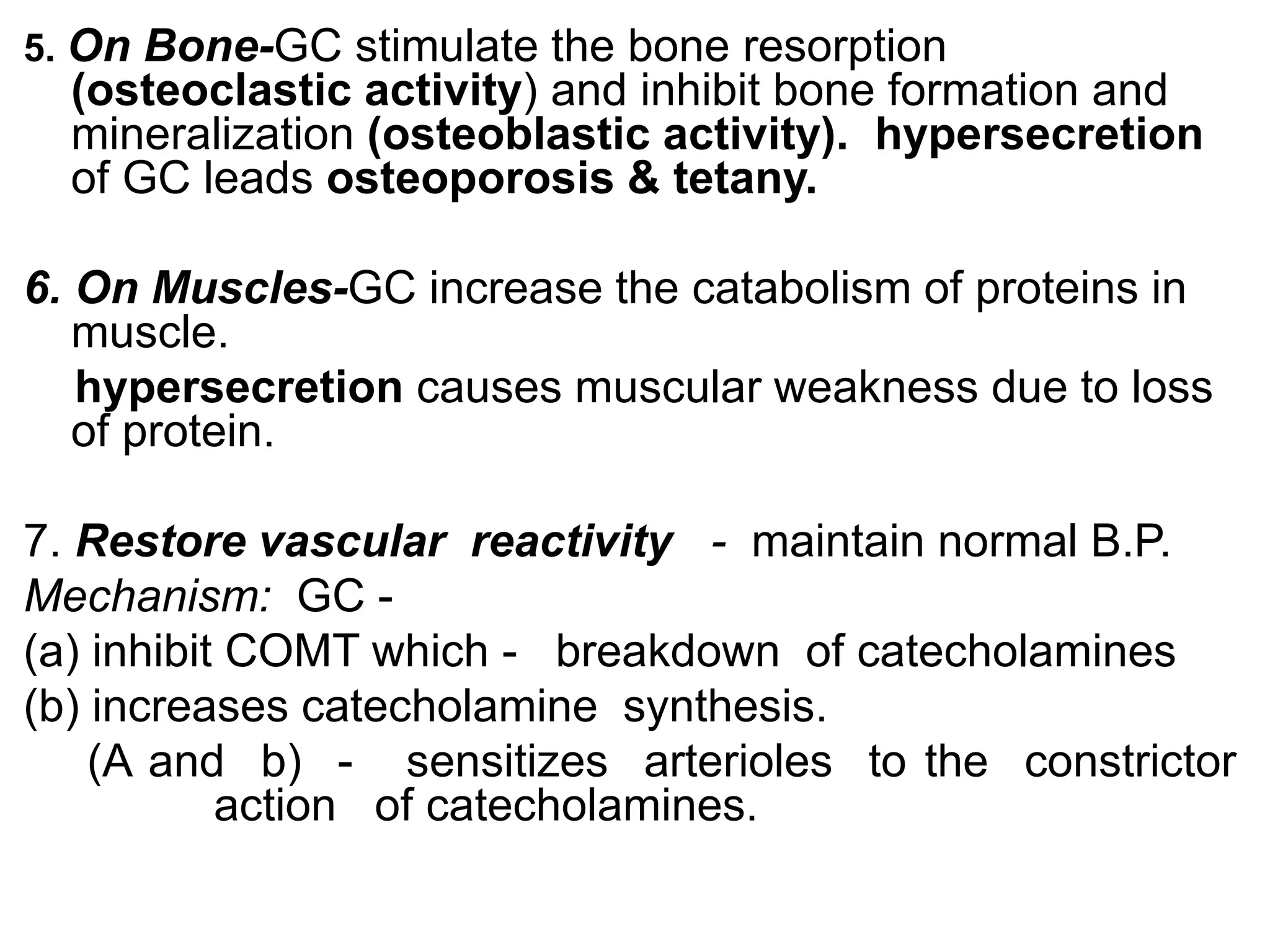
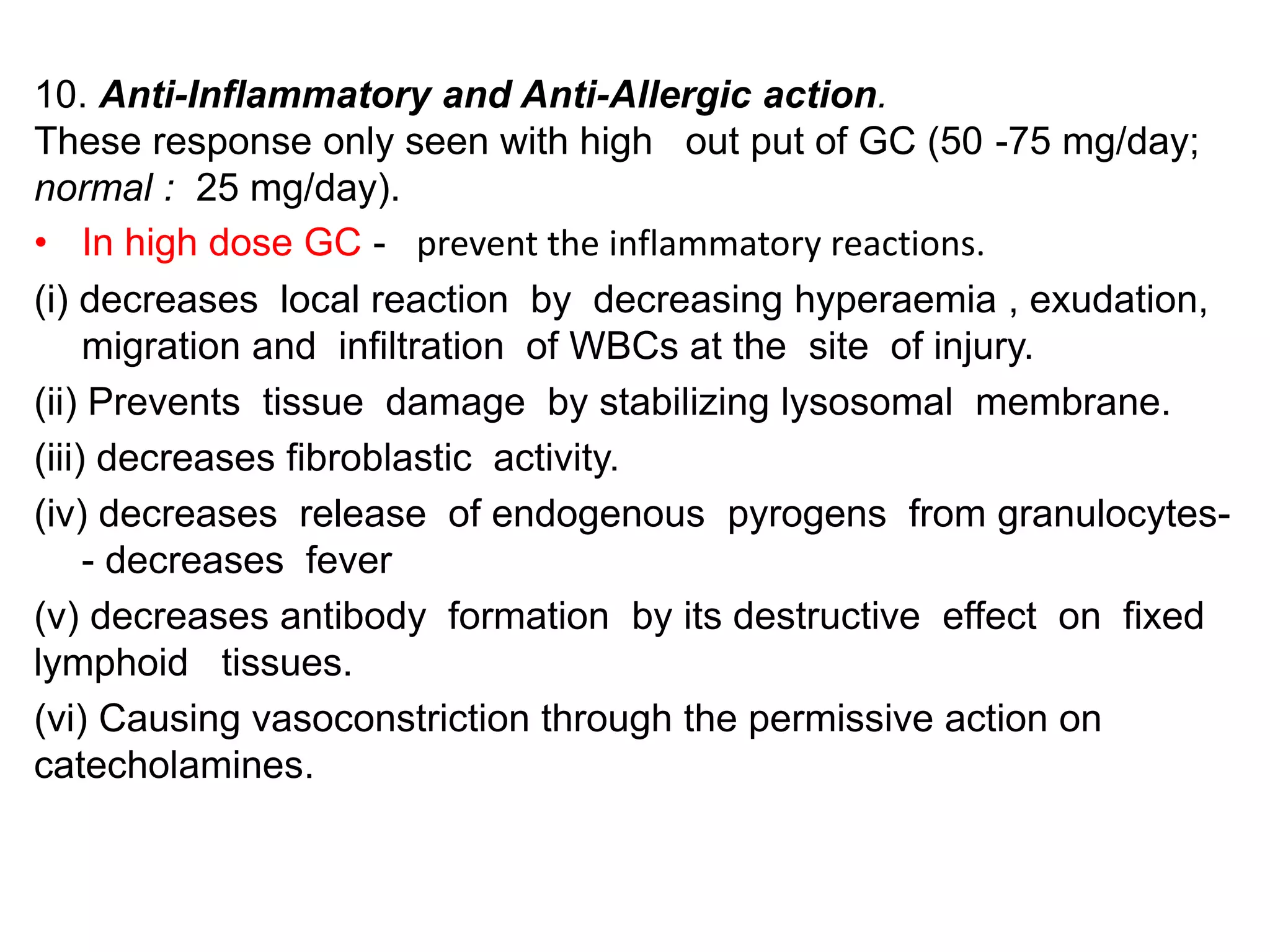
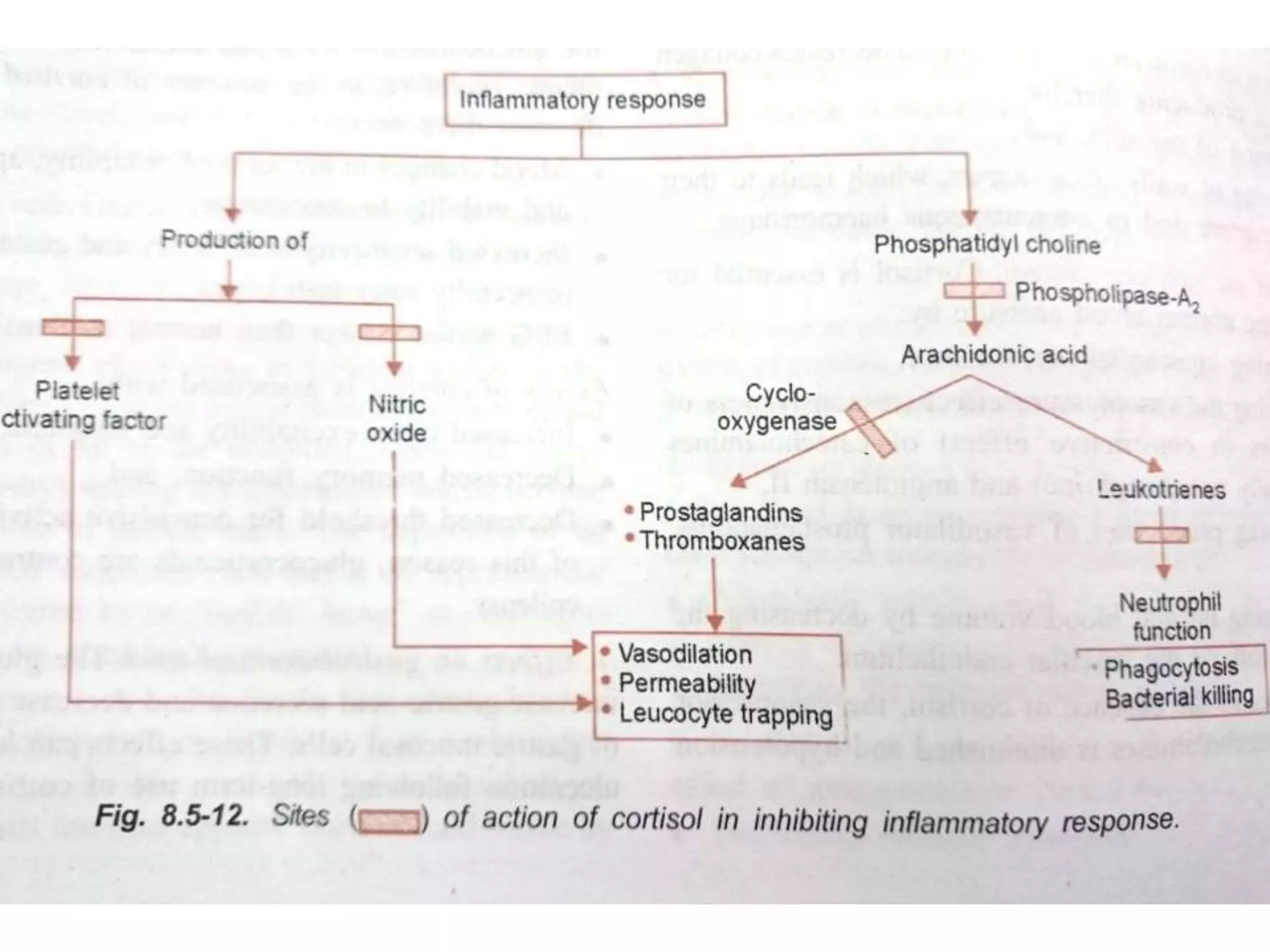
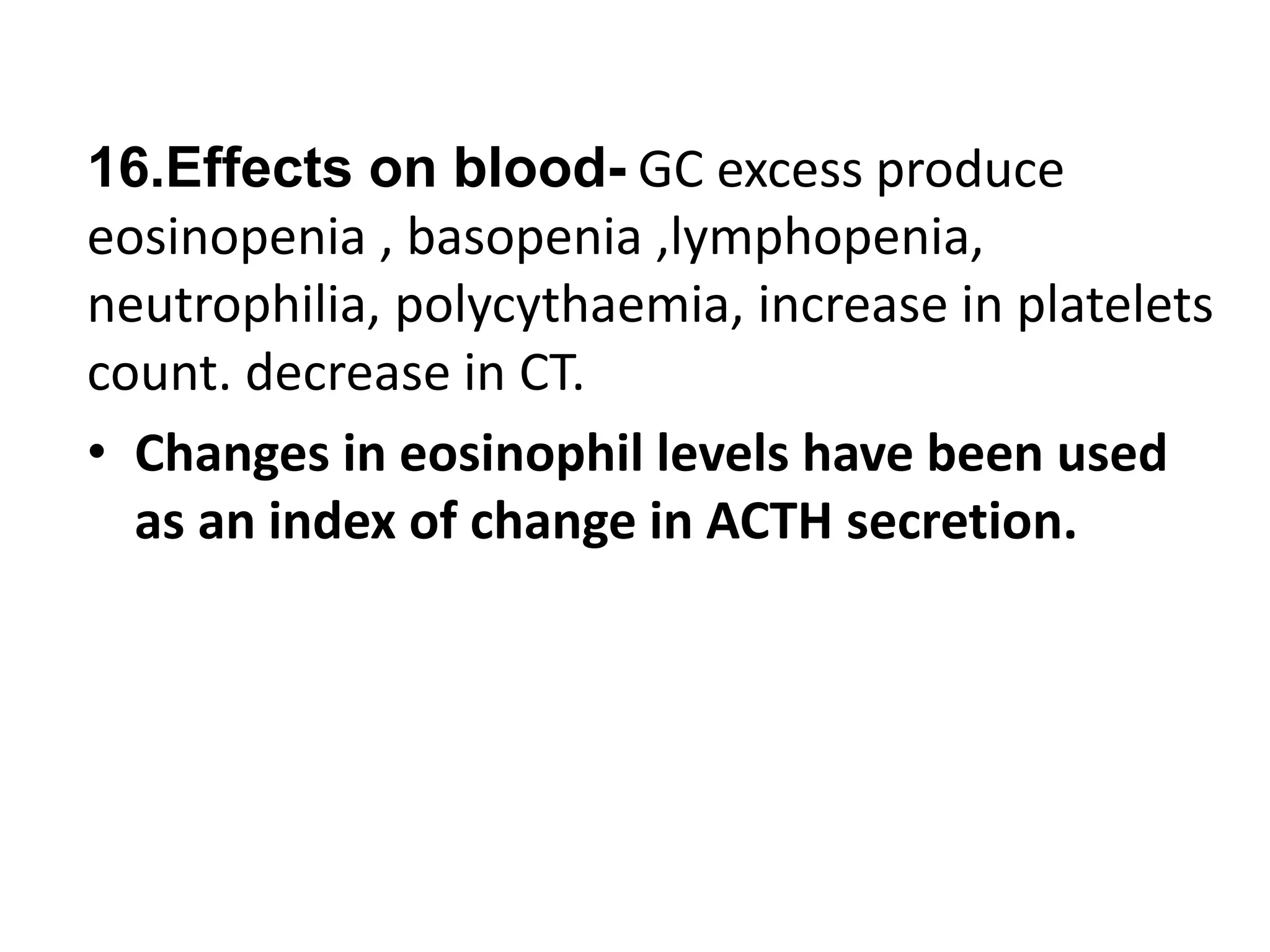
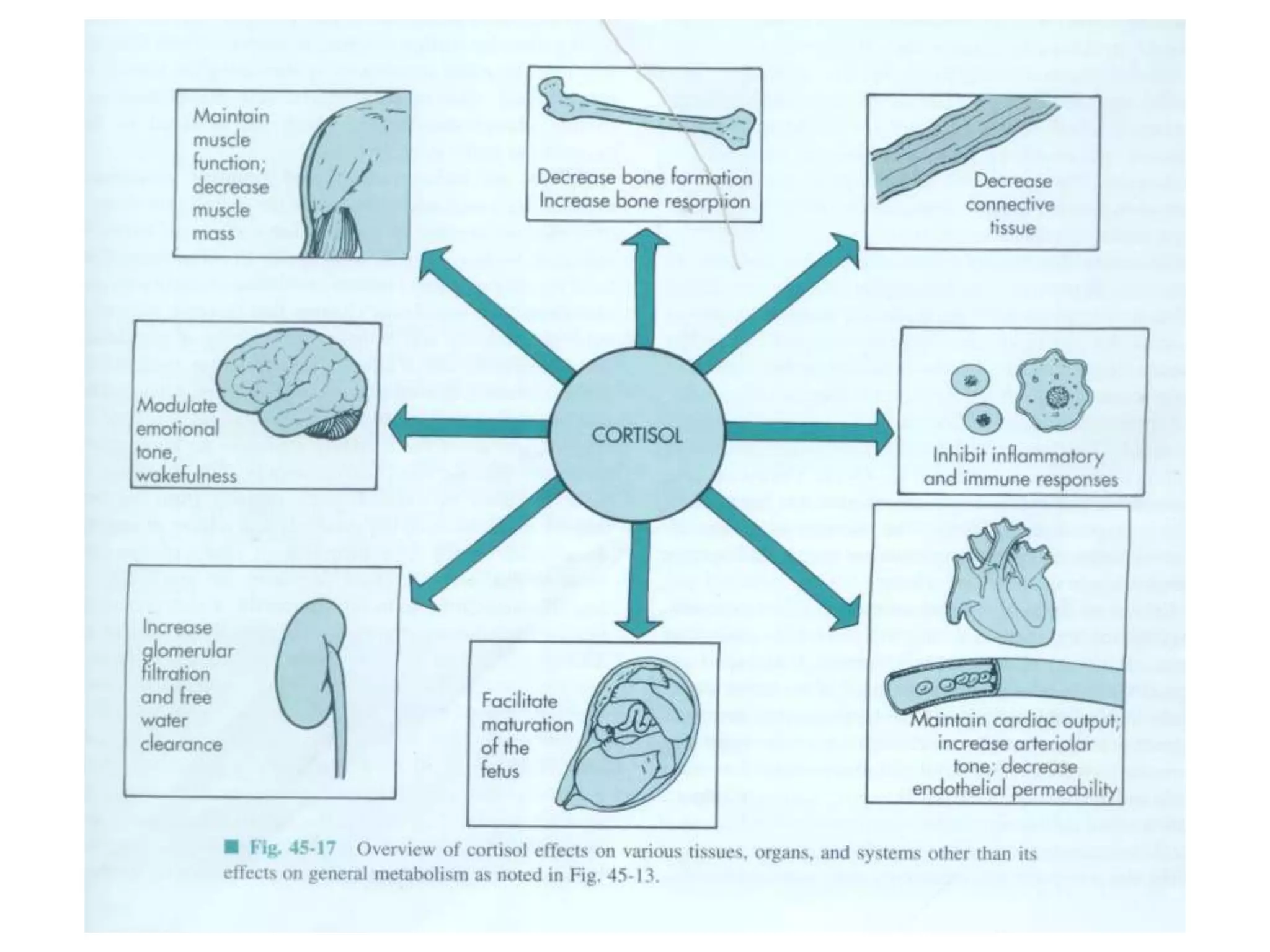
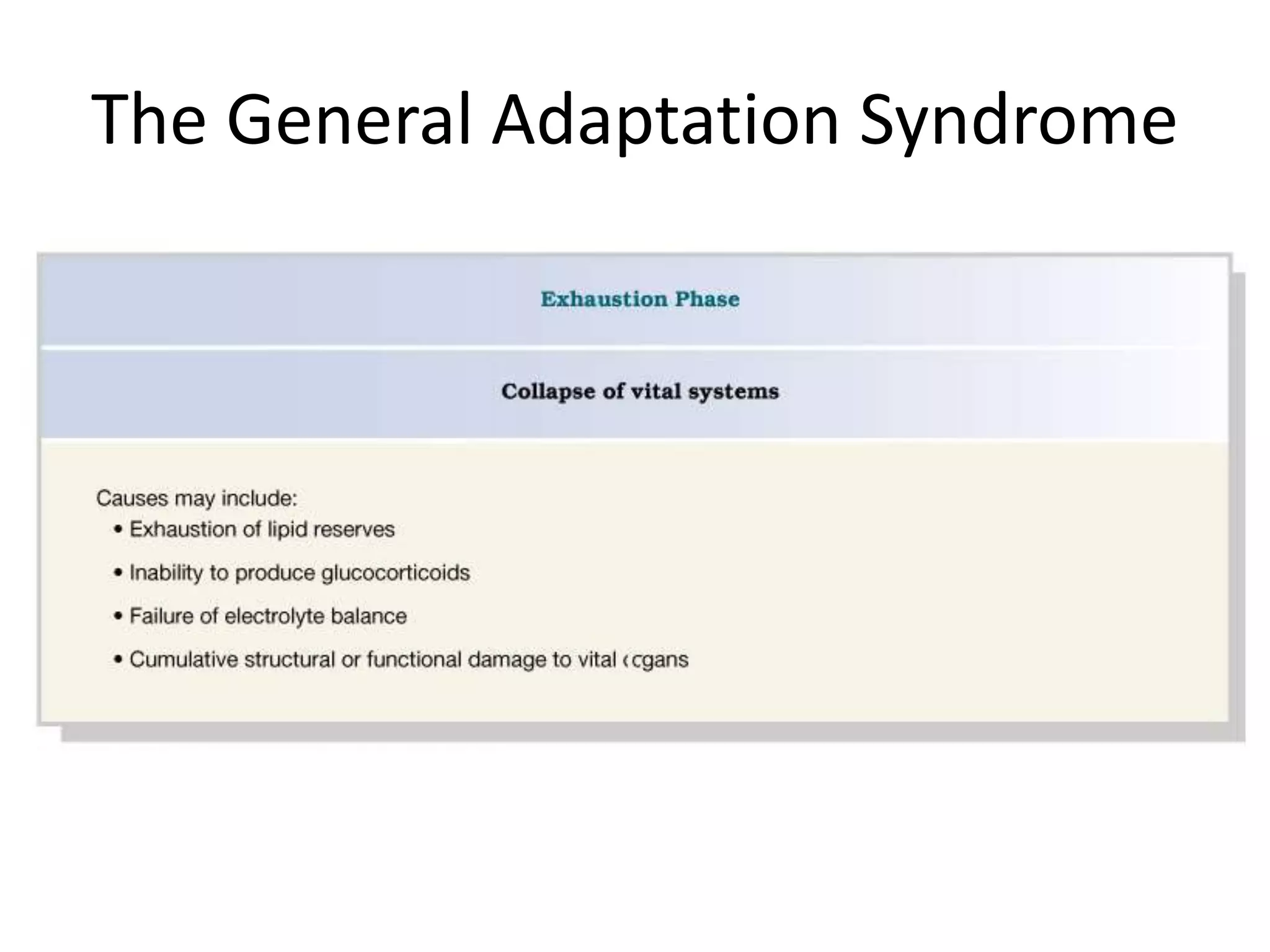
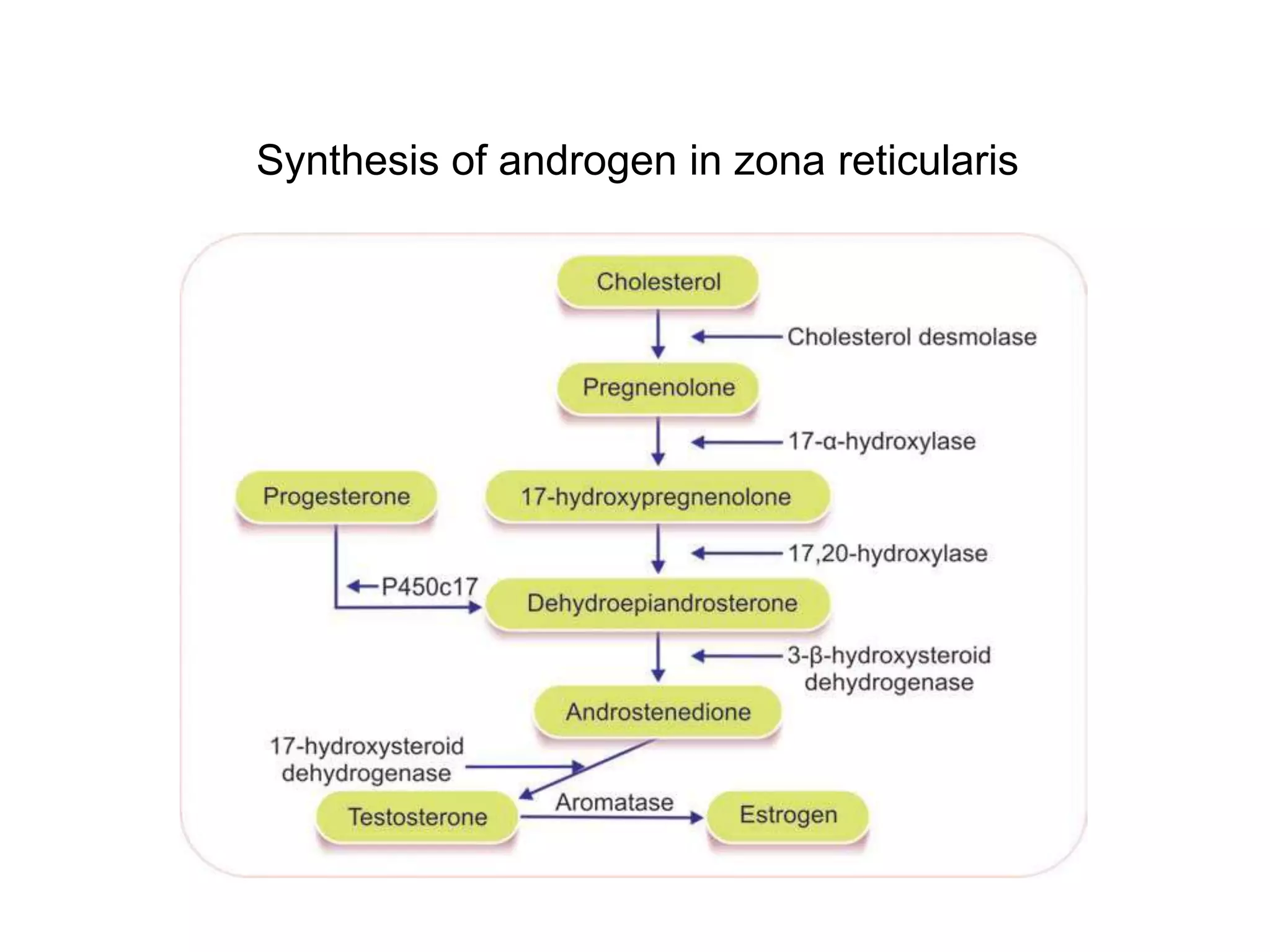
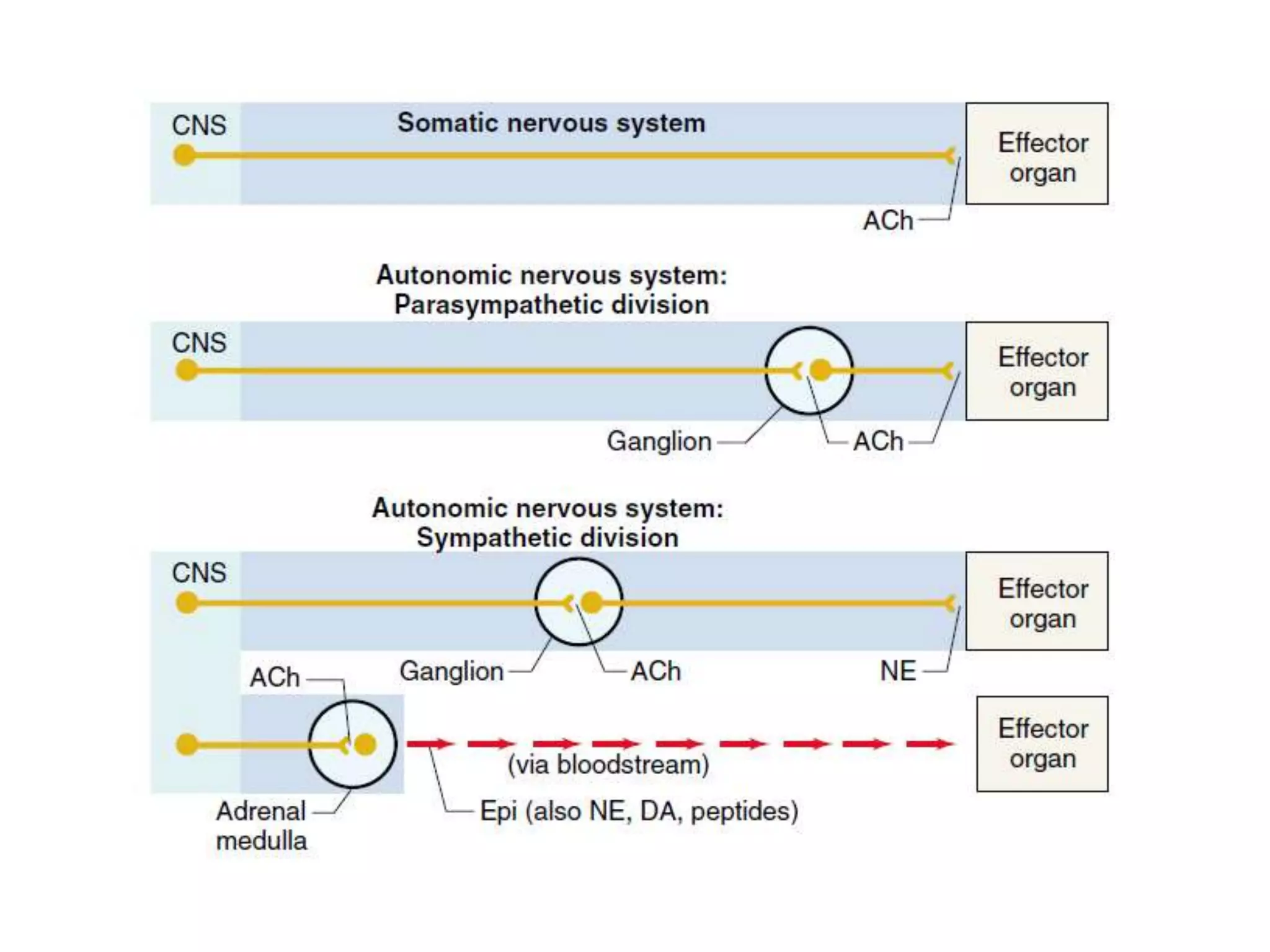
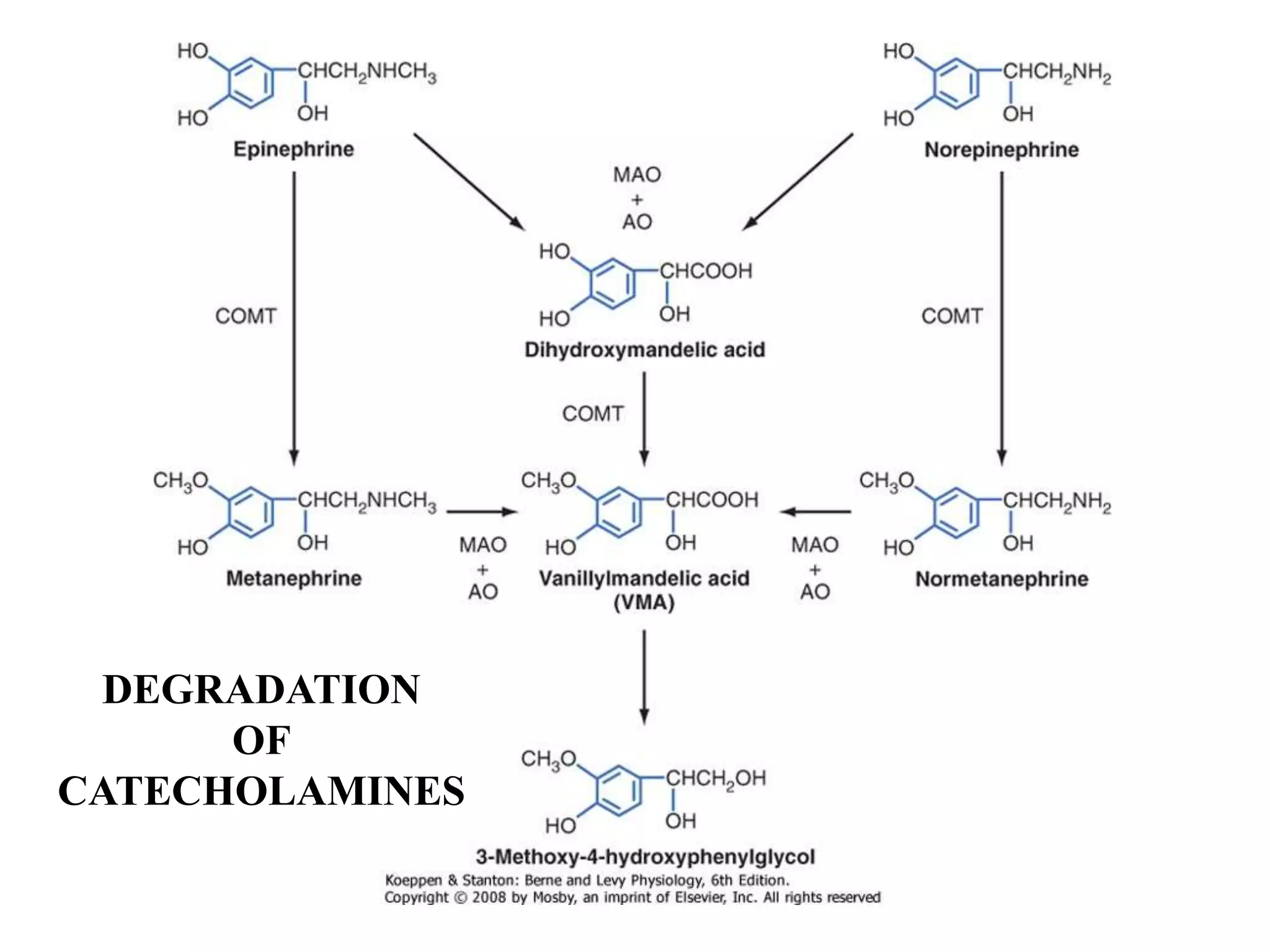
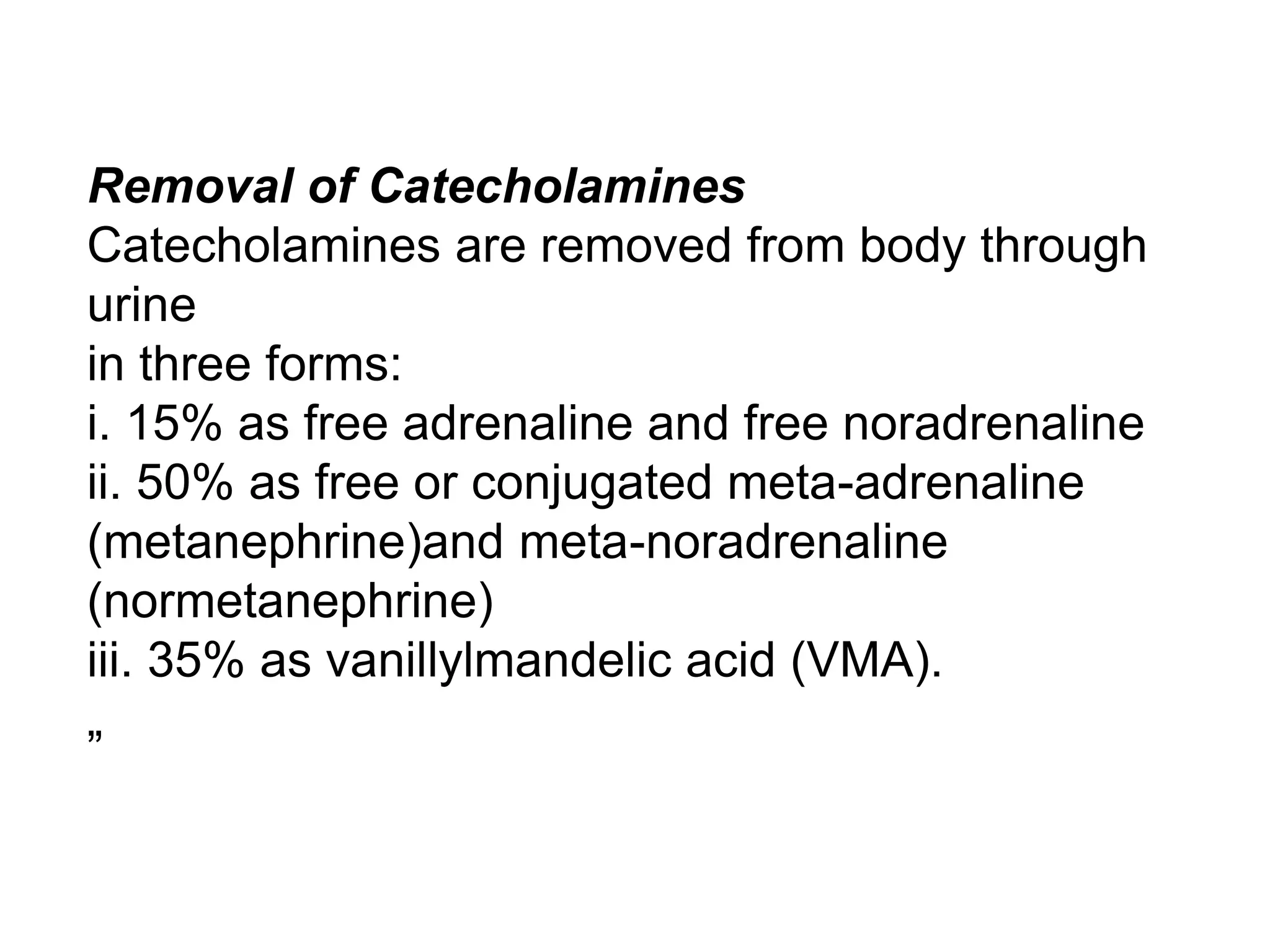
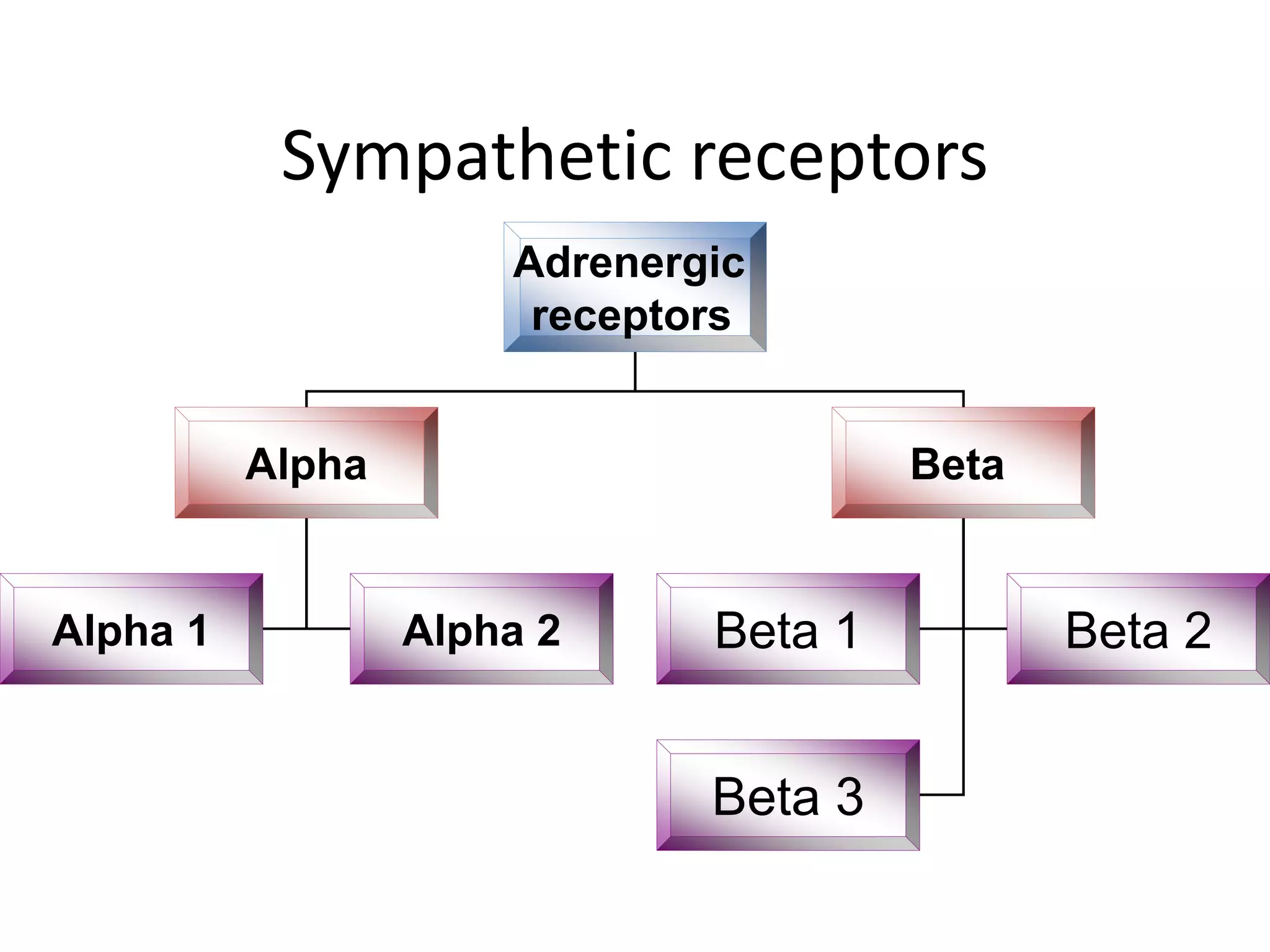
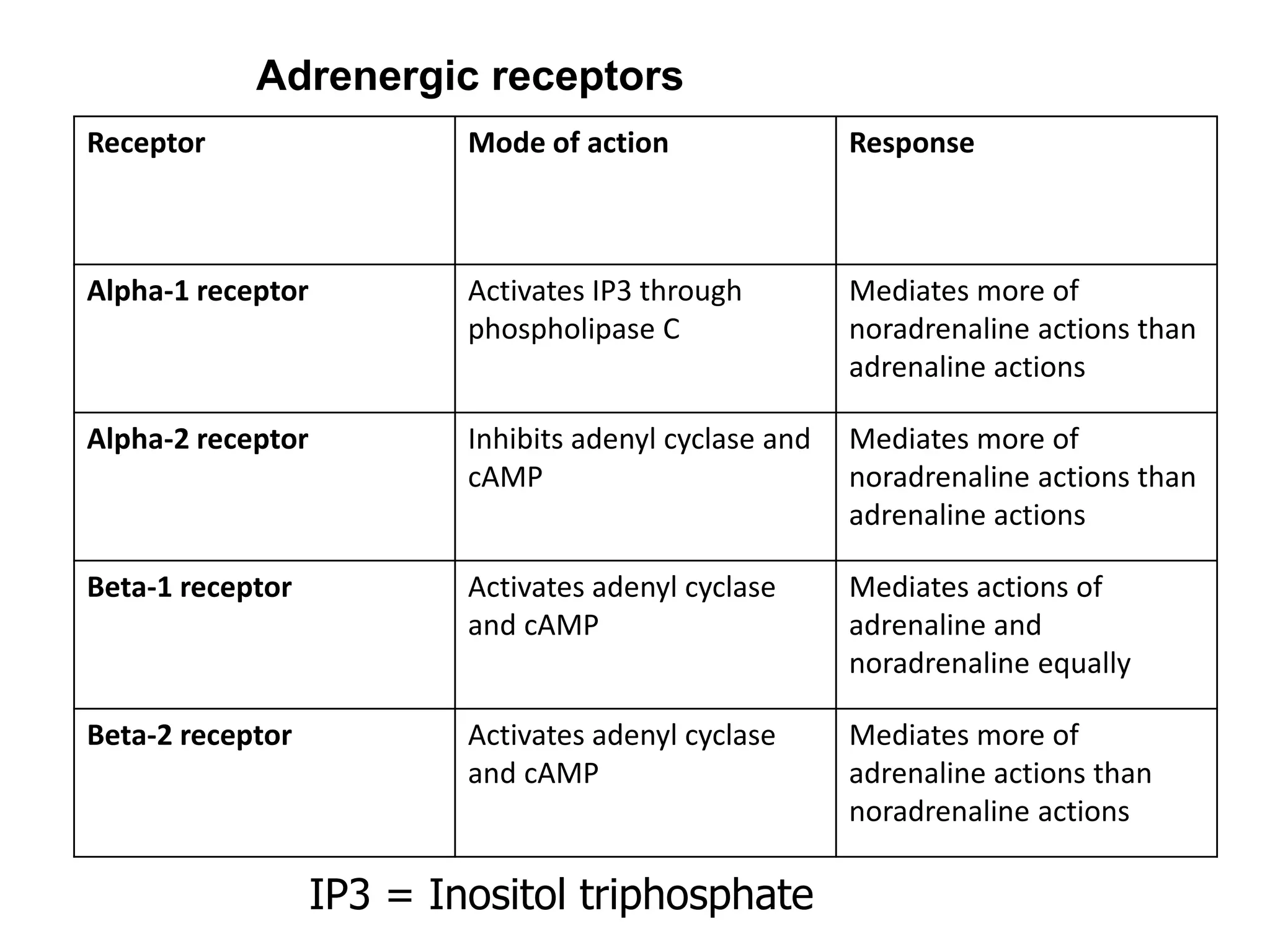
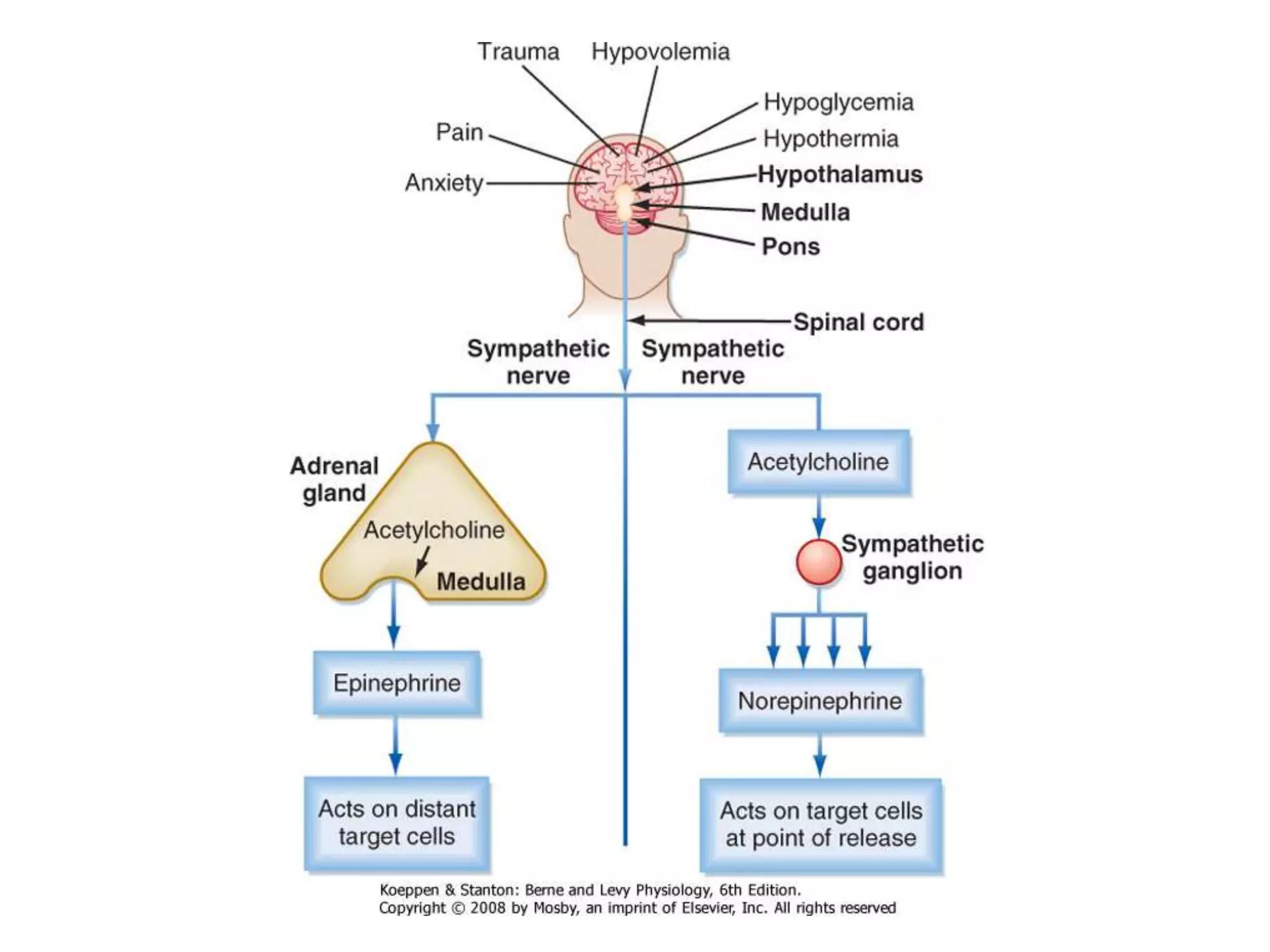
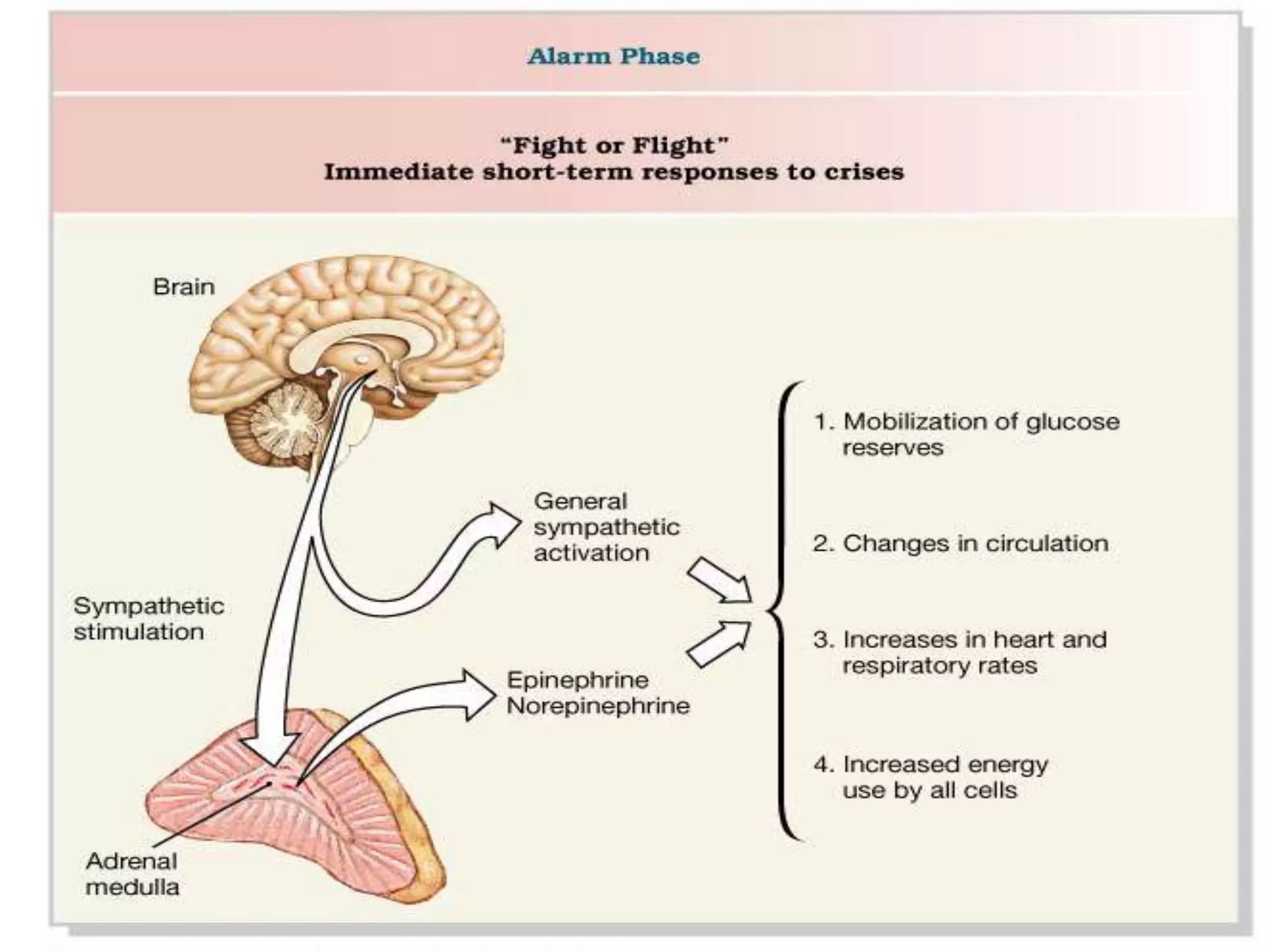
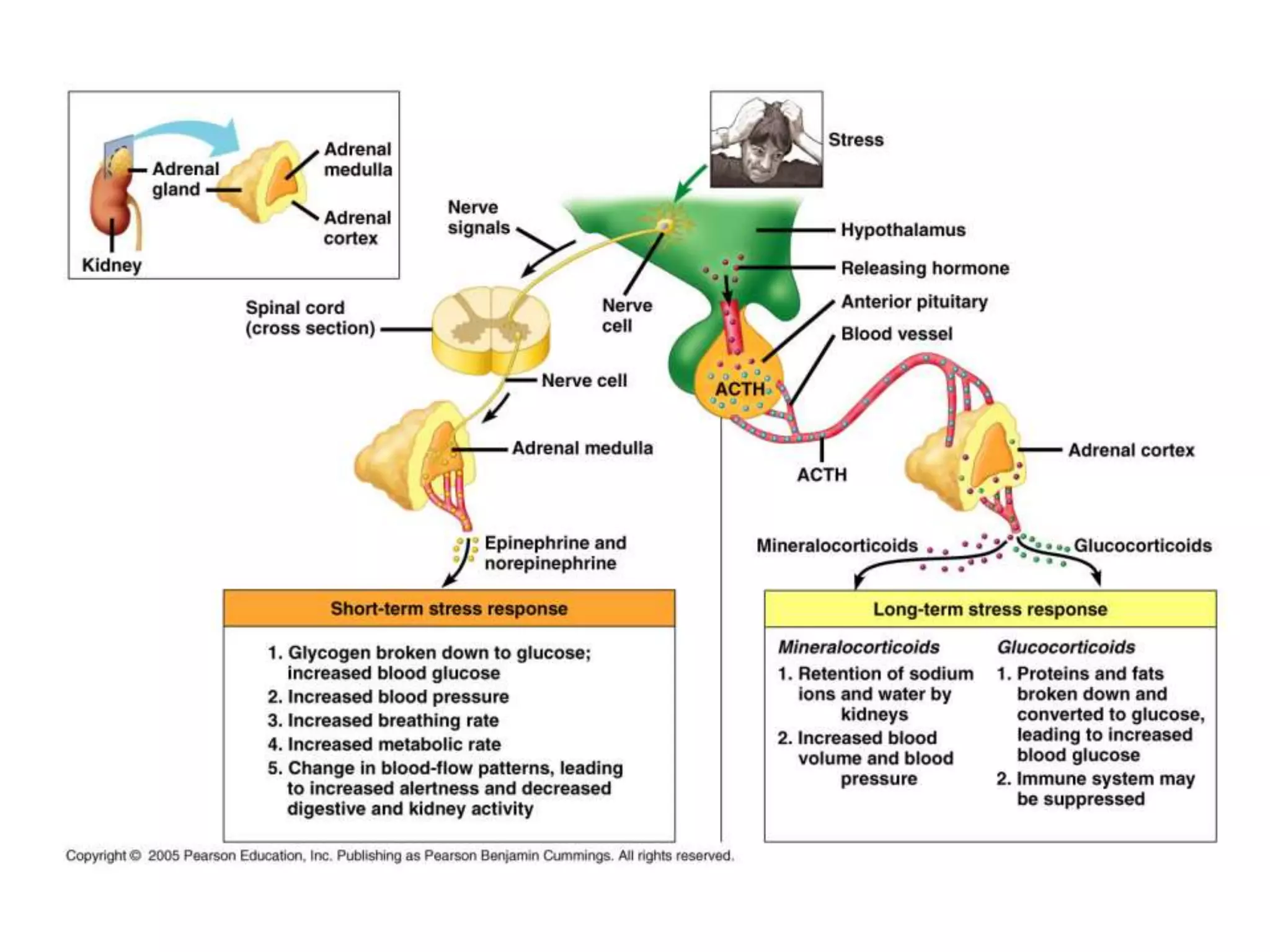
The adrenal glands are essential endocrine glands that secrete hormones that regulate fluid balance and the body's stress response. They consist of the adrenal cortex and adrenal medulla. The adrenal cortex secretes corticosteroids including mineralocorticoids like aldosterone and glucocorticoids like cortisol. Aldosterone regulates sodium and potassium levels in the body to control blood pressure and volume. Cortisol regulates carbohydrate, fat, and protein metabolism and helps the body respond to stress. Dysfunction of the adrenal cortex can cause hypo- or hypersecretion of hormones with signs and symptoms like hypertension, hypokalemia, and adrenal crisis.