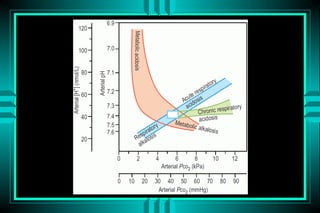
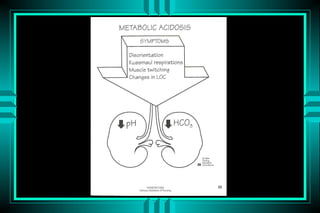
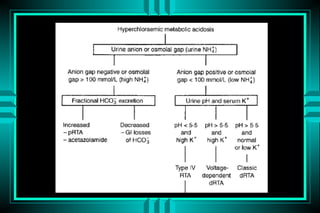
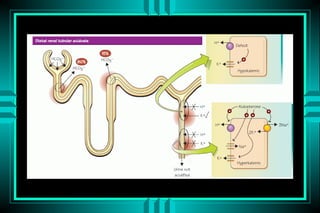
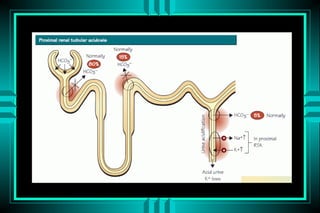
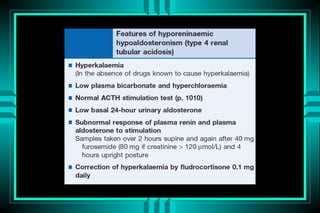
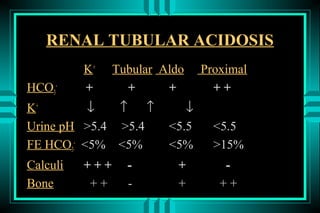
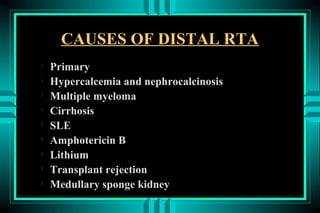
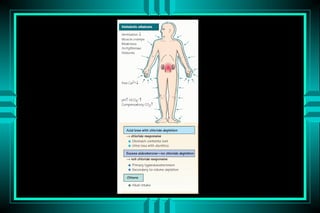
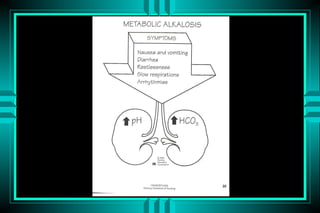
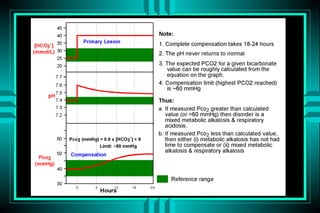
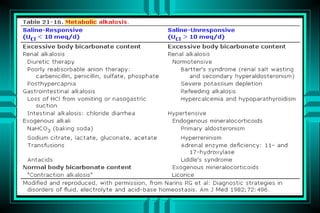
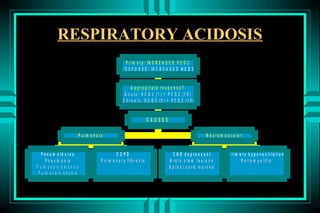
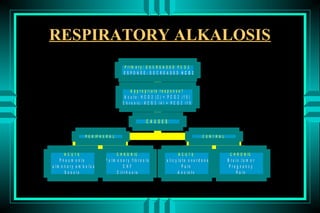
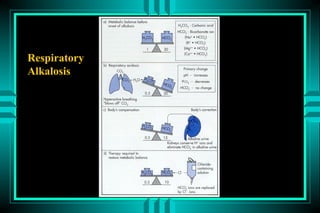
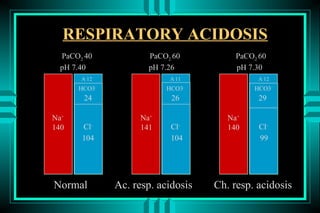
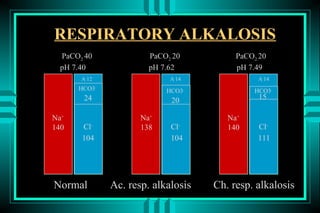
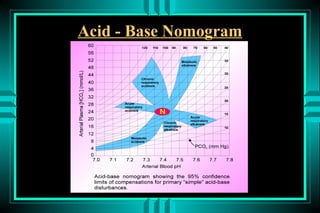
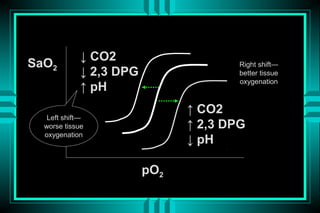
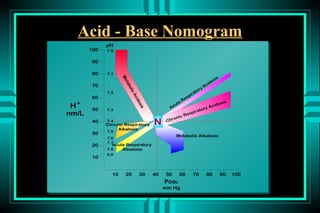
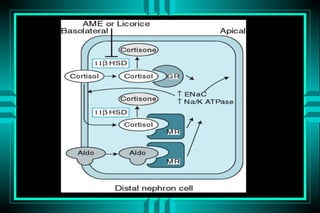
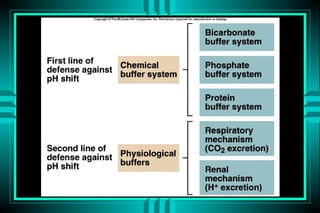
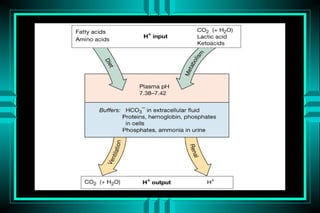
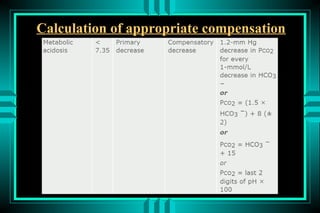
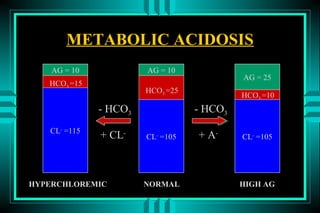
The document discusses metabolic acidosis, defining it as a primary decrease in bicarbonate with a compensatory decrease in PCO2. It notes the causes can include GI or renal bicarbonate loss, lactic acidosis, ketoacidosis from diabetes or alcohol, intoxication from ethylene glycol or methanol, and advanced renal failure. Metabolic acidosis is classified as having a normal or high anion gap, with high anion gap causes including ketoacidosis, lactic acidosis, and certain intoxications.








![ACID-BASE DISORDERS
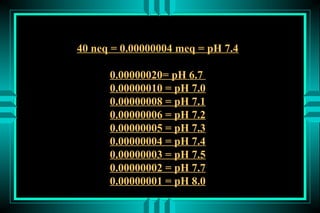
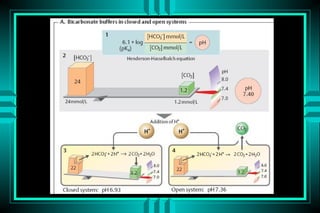
FORMULAS:
HENDERSON-HASSELBALCH
pH = pK + log ([HCO3-]/[0.03* PCO2])
pH = 6.10 + log (24/0.03*40) = 7.40
MODIFIED HENDERSON
[H+] = 24* PCO2/[HCO3-]
[H+] = 24* (40/24) = 40 neq/L (pH=7.4)](https://image.slidesharecdn.com/acid-base-2013-130203032247-phpapp02/85/Acid-base-disorders-17-320.jpg)













![ACID-BASE DISORDERS
EXAMPLE OF A SIMPLE DISORDER
pH (7.55) = C * [HCO3-] (18 mmol/L)
PCO2 (21 mm Hg)
Step 1: pH , indicates alkalemia (Met. or Resp)
Step 2: HCO3- , indicates Resp. Alkalosis
Step 3: PCO2 , confirms Resp. Alkalosis](https://image.slidesharecdn.com/acid-base-2013-130203032247-phpapp02/85/Acid-base-disorders-37-320.jpg)

![ACID-BASE DISORDERS
EXAMPLE OF A MIXED DISORDER
pH (7.55) = C * [HCO3-] (30 mmol/L)
PCO2 (35 mm Hg)
Step 1: Alkalemia
Step 2: HCO3- , indicates Met. alkalosis
Step 3: PCO2 , indicates Resp. alkalosis
Step 4: ∆ HCO3- (25%) > ∆ PCO2 (12.5%)
Step 5: The major disorder is metabolic alkalosis](https://image.slidesharecdn.com/acid-base-2013-130203032247-phpapp02/85/Acid-base-disorders-39-320.jpg)