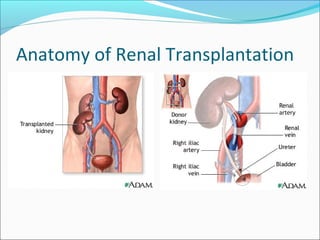
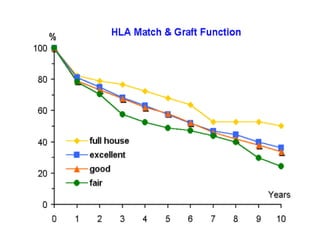
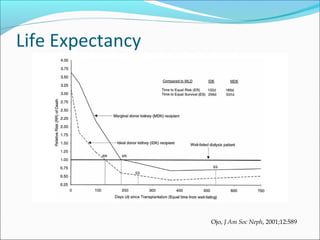
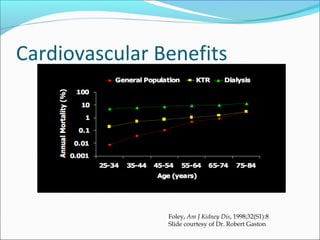
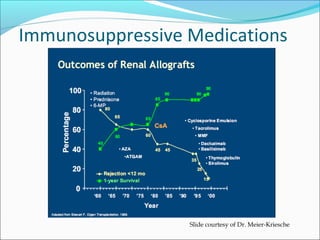
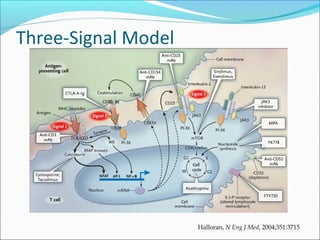
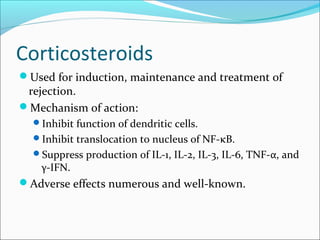
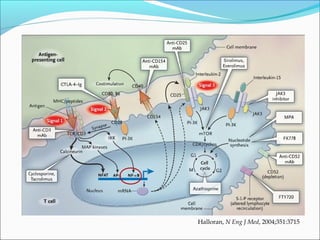
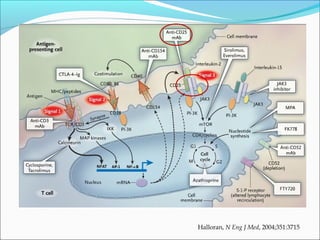
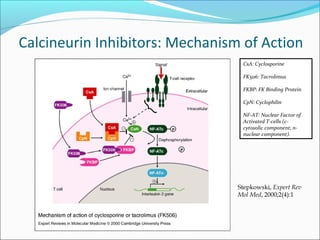
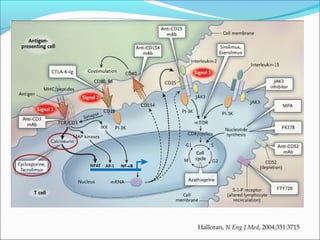
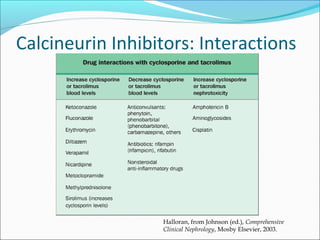
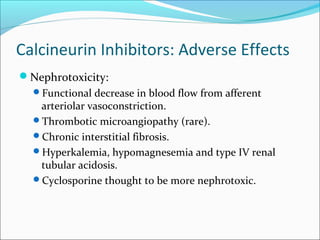
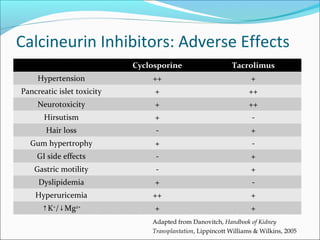
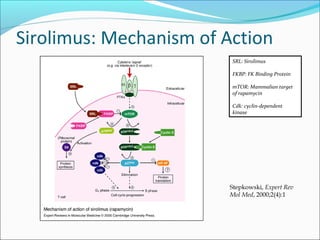
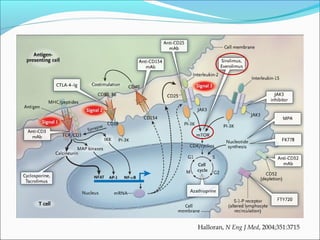
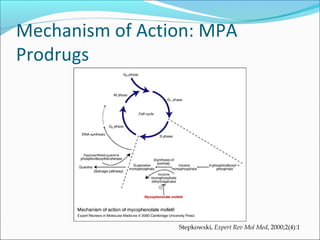
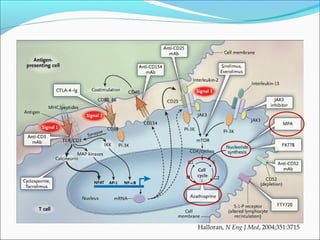
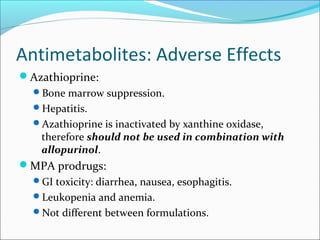
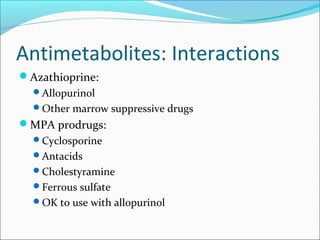
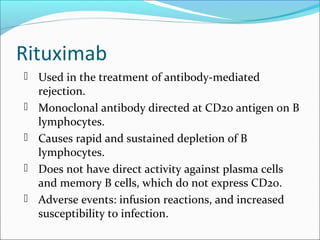
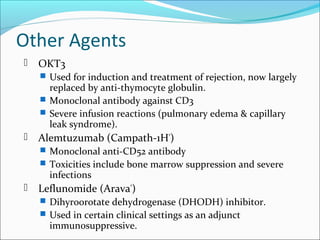
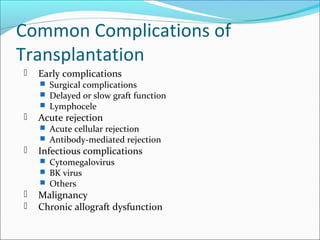
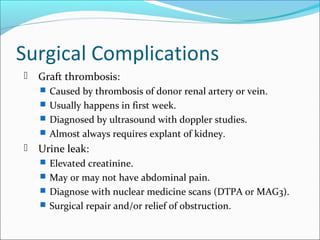
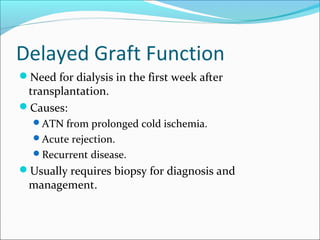
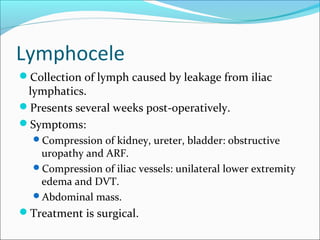
Transplantation involves implanting non-self tissue into the body from a donor to a recipient. Kidney transplantation is the most effective therapy for end-stage renal disease, with organs coming from live or deceased donors. Patients require lifelong immunosuppressive medications including corticosteroids, calcineurin inhibitors, mTOR inhibitors, and antimetabolites to prevent rejection. Common post-transplant complications include surgical complications, delayed graft function, infection, acute rejection, and chronic allograft dysfunction.