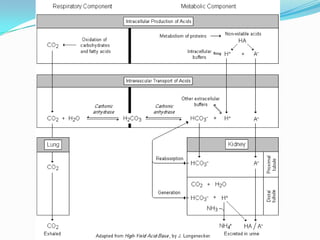
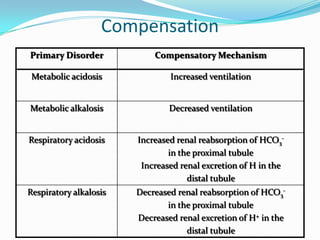
This document provides information on arterial blood gas analysis, including contraindications for arterial puncture, reasons to order an ABG, normal values, equations, and approaches to interpreting ABG results. It discusses how to determine if a patient has acidosis or alkalosis, whether it is respiratory or metabolic, and if the compensation is adequate. It provides steps to classify the acid-base disorder, consider anion and osmolal gaps, and evaluate for mixed disorders. Causes and treatments of different acid-base imbalances are outlined.




![EQUATIONS
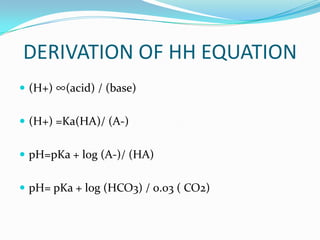
Henderson Hasselbalch equation: pH = 6.1 + log [HCO3-]
0.03( Pco2)
Kassirer-Bleich equation: [H+] = 24 × PCO2 / [HCO3-]](https://image.slidesharecdn.com/abganalysis-111230000415-phpapp02/85/ABG-APPROACH-6-320.jpg)
![HYDROGEN ION CONC AT dif PH
pH [H+]
7.7 20
7.5 31
7.4 40
7.3 50
7.1 80
7.0 100
6.8 160](https://image.slidesharecdn.com/abganalysis-111230000415-phpapp02/85/ABG-APPROACH-8-320.jpg)





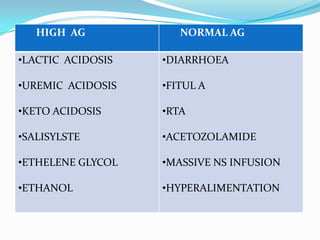
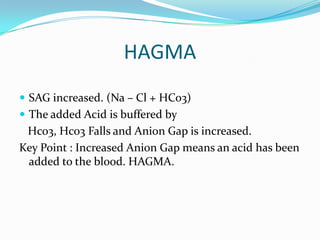
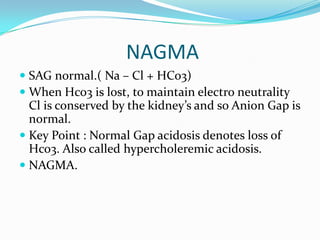
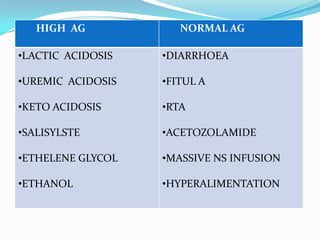

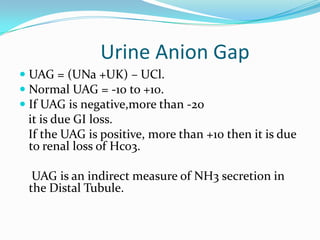
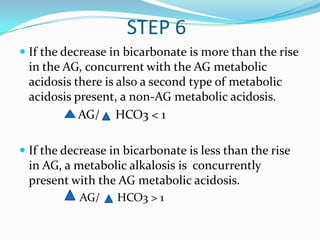


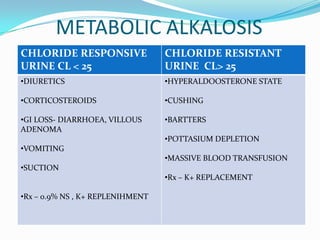
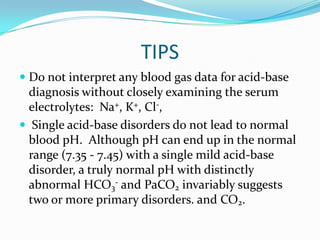
![TIPS
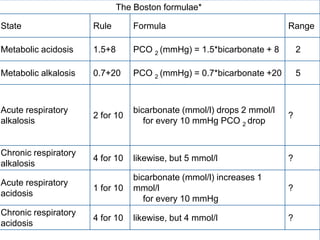
Simplified rules predict the pH and HCO3- for a
given change in PaCO2. If the pH or HCO3- is
higher or lower than expected for the change in
PaCO2, the patient probably has a metabolic acid-
base disorder as well.
In maximally-compensated metabolic acidosis, the
numerical value of PaCO2 should be the same (or
close to) as the last two digits of arterial pH. This
observation reflects the formula for expected
respiratory compensation in metabolic acidosis:
Expected PaCO2 = [1.5 x serum hco3] + (8 ± 2)](https://image.slidesharecdn.com/abganalysis-111230000415-phpapp02/85/ABG-APPROACH-27-320.jpg)






