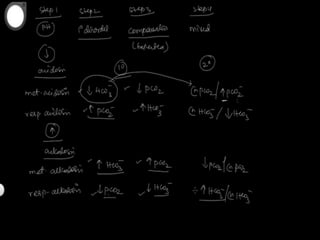
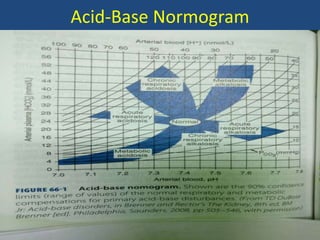
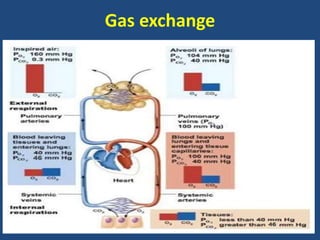
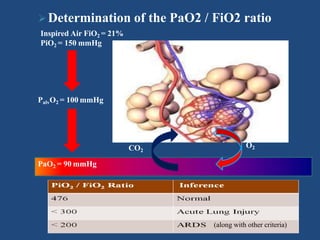
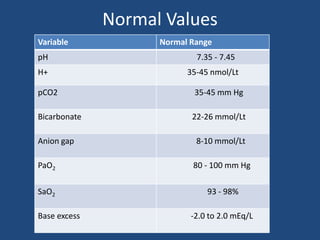
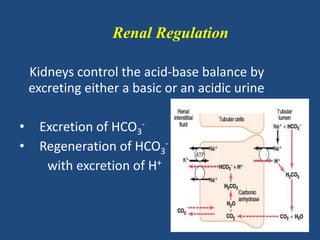
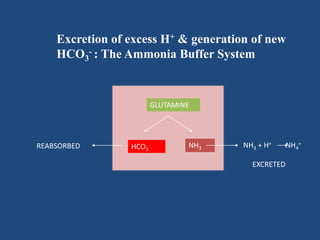
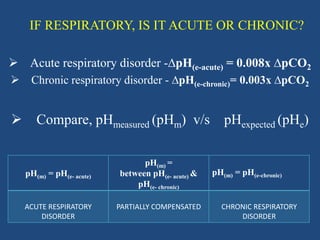
The document discusses arterial blood gas analysis and interpretation. It provides an overview of gas exchange, acid-base homeostasis, and the basics of acid-base balance. It describes how to interpret an arterial blood gas report, including how to diagnose acid-base disorders and examples. Technical aspects like sampling technique and potential errors or complications are covered. Compensation mechanisms in response to primary acid-base disturbances are explained.
















![Buffers
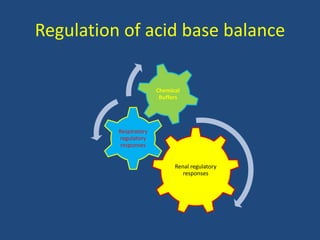
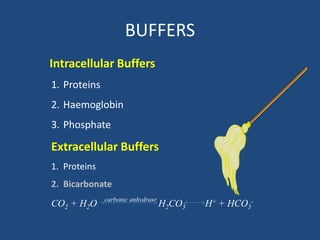
• Buffers are chemical systems which either
release or accept H+ and minimize change in
pH induced by an acid or base load.
• First line of defense blunting the changes in
[H+]
• A buffer pair consists of: A base (H+ acceptor)
& An acid (H+ donor)
• Extracellular and Intracellular Buffer.](https://image.slidesharecdn.com/abgbydrpratapsingh-190319193422/85/ABG-Analysis-21-320.jpg)



![Compensatory changes (Metabolic disorders)
Primary
disorder
Primary
defect
Compensatory
response
Expected Compensation
Metabolic
acidosis
↓ HCO3 ↓ PCO2 PCO2=1.5[HCO3] + 8 ± 2
PCO2= PaCO2 will ↓ 1.25 mmHg per mmol/L ↓ in
HCO3
PCO2= 15+ [HCO3]
Metabolic
Alkalosis
↑ HCO3 ↑ PCO2 PCO2=1.5[HCO3] + 8 ± 2
PCO2= PaCO2 will ↑ 0.75 mmHg per mmol/L↑
in HCO3
PCO2=15+ [HCO3]](https://image.slidesharecdn.com/abgbydrpratapsingh-190319193422/85/ABG-Analysis-30-320.jpg)










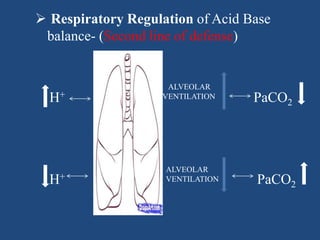
![RESPIRATORY ACIDOSIS
H2O + CO2 H2CO3 H+ + HCO3
-
Cause - hypoventilation
Retention of CO2
Drives equation rightward
Increases both [H+] and [HCO3
-]](https://image.slidesharecdn.com/abgbydrpratapsingh-190319193422/85/ABG-Analysis-42-320.jpg)


![RESPIRATORY ALKALOSIS
H2O + CO2 H2CO3 H+ + HCO3-
cause - hyperventilation
Blows off CO2
Drives equation leftward decreasing both [H+] and [HCO3
-]](https://image.slidesharecdn.com/abgbydrpratapsingh-190319193422/85/ABG-Analysis-45-320.jpg)













![ Osmolar Gap
• The Osmolar gap is used to detect the presence of ingested toxins
such as ethylene glycol, methanol or isopropyl alcohol
• These Toxins often cause an increased AG acidosis. The Osmolar gap
is the difference between the measured osmolality and the
calculated osmolality
• The calculated osmolality is determined by 2*[Na] + Serum
Glucose/18 + BUN/2.8
• An Osmolar gap >15mOsm suggests the presence of an ingested toxin
as a contributor to the anion gap acidosis
Urinary Anion Gap
Used to differentiate between Renal and Extra-renal cause of
normal anion gap metabolic acidosis
It Represent unmeasured Anion in Urine like Sulfate,phosphate
Indirect estimation of Urinary Ammonium Excretion
Urinary Anion Gap = UNa + Uk-Ucl
Normal Value -10 to +10](https://image.slidesharecdn.com/abgbydrpratapsingh-190319193422/85/ABG-Analysis-60-320.jpg)