The document discusses acid-base imbalance, defining acids and bases while emphasizing the importance of maintaining physiological pH levels for vital bodily functions. It outlines the sources of hydrogen ion production and the mechanisms of pH regulation through buffering systems, respiratory, and renal processes. The text also classifies acid-base imbalance into acidosis and alkalosis, detailing their types, causes, and compensatory mechanisms.


















![ACIDOSIS
A. Metabolic Acidosis
• It is the commonest disturbance of acid-base balance
observed clinically.
• It is caused when there is a reduction in the plasma HCO3–
↓ with either no or little change in the H2CO3 fraction.
Mechanisms:
If primary deficit of HCO3– occurs the ratio [HCO –3 ]/ [H2
CO3] is decreased, i.e. pH is decreased resulting in metabolic
acidosis (primary bicarbonate deficit).
•](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-26-320.jpg)
![COMPENSATORY MECHANISM
(a)Primary compensatory mechanism (Respiratory)
• The respiratory centre is stimulated by acidosis causing deep
and rapid breathing (increased ventilation).
• This increased ventilation will result in CO2 loss and
reduction in [H2CO3]
↓ (carbonic acid).
• As a result, the ratio of [HCO–3]/[H2CO 3] is restored as
levels of both in blood are reduced.
NOTE: increased ventilation causes reduction in pCO2 ↓,
which in turn depresses the respiratory centre](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-27-320.jpg)

![DIAGNOSIS AND BIOCHEMICAL
CHARACTERISTICS
(a)Uncompensated:
If uncompensated, it is characterized biochemically in
plasma or blood as follows:
• Disproportionate decrease in [HCO –3 ] ↓
• Decrease in [H 2CO 3 ] ↓ and pCO2 ↓
• Decrease in total CO 2 content [HCO –3] + [H 2 CO 3]
• Decrease in [HCO–3 ] : [H2CO 3 ] ratio ↓
• Decrease in pH ↓](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-29-320.jpg)
![(b) Fully compensated:
• If fully compensated the CO2 content is low
• Decrease in [HCO –3 ] and [H2CO3] is proportionate,
• [HCO–3 ] : [H2CO 3] ratio remain within normal
limits.
• pH remain within normal limits.](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-30-320.jpg)




![B. RESPIRATORY ACIDOSIS
• It is also called as “primary [H 2CO 3] carbonic acid
excess’’.
• The underlying abnormality here is increase in H2CO3 in
the blood, which follows decreased elimination of CO2 in
the pulmonary alveoli and result in increase in (pCO2 ↑)
This may result from:
1. Breathing air containing abnormally high percentage of
CO 2
2. Conditions in which elimination of CO2 through lungs is
retarded](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-35-320.jpg)
![MECHANISM
• If excretion of CO2 through lungs is impaired
(e.g. emphysema or depression of respiratory centre),
more CO 2 will accumulate in blood, resulting in excess
H2CO3 formation [H 2CO 3 ] ↑.
• This results in lowering the ratio of [HCO –3]/[H 2 CO 3 ],
resulting lowering in pH ↓and is described as “Respiratory
acidosis” (carbonic
acid excess).](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-36-320.jpg)
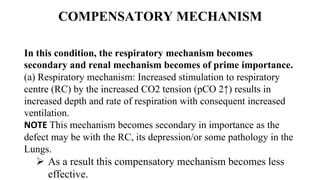
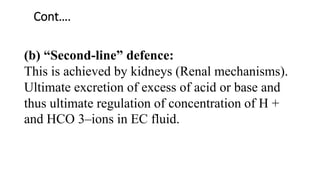
![(b) Renal mechanism: It is of prime
importance. More HCO –3 are reabsorbed
from tubules in response to raised pCO 2 in
blood and ratio of [HCO–3 ]/[H2CO 3] is
restored as the levels of both in blood are
increased.](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-38-320.jpg)
![BIOCHEMICAL/DIAGNOSTIC
CHARACTERISTICS
(a) If uncompensated, it is characterised biochemically
(plasma or blood) as follows:
• Disproportionate increase in [H 2CO 3] ↑ (pCO 2) ↑
• Increase in [HCO–3 ] ↑
• Increase in total CO 2 content ↑
• Decrease in [HCO–3 ] : [H 2CO 3] ratio ↓
• Decrease in pH ↓](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-39-320.jpg)
![(b) If fully compensated,
• CO 2-content is high
Increase in [H2 CO 3] and [HCO –3] are
proportionate,
• [HCO –3 ] : [H2 CO 3] ratio remaining within
normal limits.
• pH remaining within normal limits.](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-40-320.jpg)






![ALKALOSIS
A. Metabolic Alkalosis
• Also called as primary alkali excess.
• This condition results from an absolute or relative increase in [HCO
3].
Mechanism
1. Excess of HCO 3 accumulation (soluble alkali ingestion) causes
an increase in the ratio of [HCO3–]/[H2CO 3] (i.e. pH is increased
↑) and it is known as “Metabolic alkalosis” (“bicarbonate
excess”).](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-47-320.jpg)
![2. The respiratory centre (RC) is inhibited by alkalosis
causing shallow, irregular breathing.
• This reduced ventilation will result in CO2 retention and increases in
carbonic acid level [H2CO 3] ↑.
3. The ratio of [HCO–3]/[H2CO3] will be restored as the levels of both
in blood are increased.
4. However, decreased ventilation raises pCO 2, which
tends to stimulate the RC.
NOTE: Raised pCO 2 stimulating the RC are working simultaneously
and the respiratory compensation is incomplete](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-48-320.jpg)
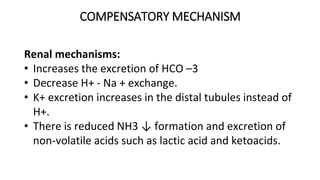
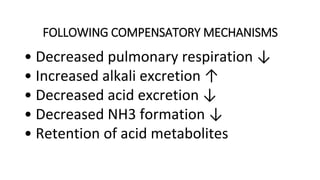
![DIAGNOSTIC/BIOCHEMICAL
CHARACTERISTICS
(a) If uncompensated phase
• Disproportionate increase in [HCO –3] ↑
• Increase in [H2 CO 3] ↑, pCO 2 ↑
• Increase in total CO 2 content
• Increase in [HCO –3 ]:[H2 CO 3] ratio ↑
• Increase in pH ↑](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-51-320.jpg)
![(b) If fully compensated
• CO 2 content is high
• Increase in [HCO–3] and [H 2 CO 3] are
proportionate
• [HCO –3 ] : [H2 CO 3] ratio remaining within
normal limits
• pH remaining within normal limits.](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-52-320.jpg)


![RESPIRATORY ALKALOSIS…
Also called as primary H2CO3 deficit.
This condition occurs when there is a decrease in [H2CO3] ↓
fraction with no corresponding change in HCO–3 in plasma.
Excessive quantities of CO2 may be washed out of the blood by
hyperventilation.
Mechanism:
Increased loss of CO2 (due to hyperventilation), results in
diminution of [H2CO3] ↓. The ratio of [HCO–3]/[H2CO3] is
increased ↑ i.e. pH is increased and is termed “respiratory
alkalosis” (carbonic acid deficit)](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-55-320.jpg)
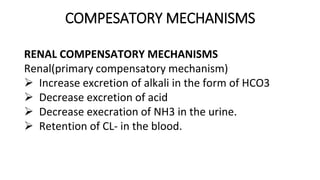
![DIAGNOSTIC AND BIOCHEMICAL
CHARACTERISTIC
a. If UNCOMPENSATED
is characterized in the plasma or blood biochemically
as follow
Disproportionate decrease in [H2CO3] and PCO2
Decrease in [HCO3]
Decrease in CO2 contents
Increase in [HCO3]; [H2CO3] ratio disproportionate.
Increase of the PH.](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-57-320.jpg)
![DIAGNOSTIC AND BIOCHEMICAL
CHARACTERISTIC….
b. If fully compensated
Low CO2 contents
Decrease in [HCO3] and [H2CO3] is proportionate
Ratio of [HCO3]:[H2CO3] remain within normal
limits.
PH remain within normal limits.](https://image.slidesharecdn.com/acidbaseimbalance2-230802105503-0d649cc4/85/ACID-BASE-IMBALANCE-2-pptx-58-320.jpg)