Embed presentation
Downloaded 10 times









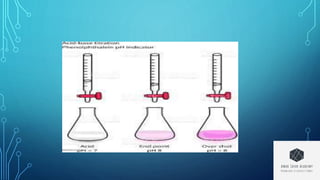


This document discusses acid-base titration and theories of acids and bases. It introduces acid-base titration as involving the neutralization reaction between an acid and base. Three theories of acids and bases are described: Arrhenius acid-base theory defines acids as producing H+ ions and bases as producing OH- ions. Bronsted-Lowry proton theory defines acids as proton donors and bases as proton acceptors. Lewis electron theory defines acids as electron pair acceptors and bases as electron pair donors. The document also discusses acid-base indicators and their use in determining the endpoint of a titration, as well as Ostwald and resonance theories of indicators.










