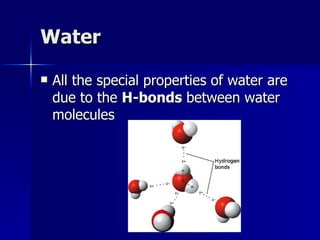
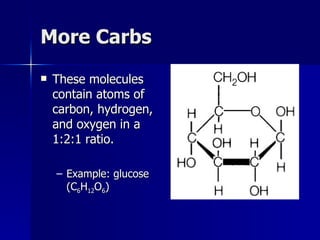
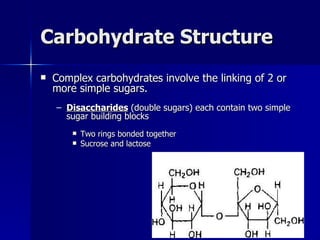
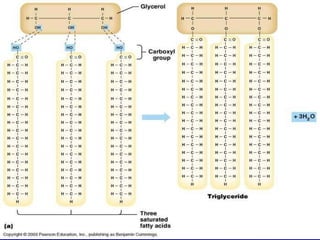
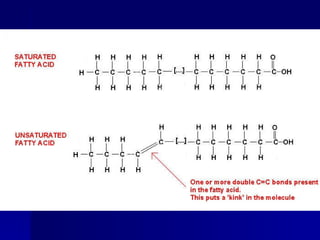
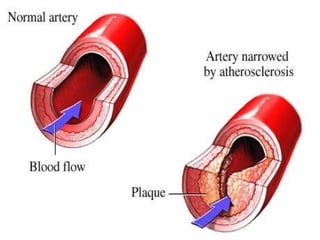
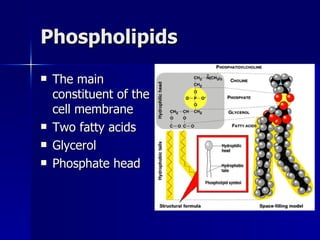
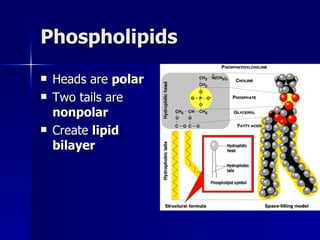
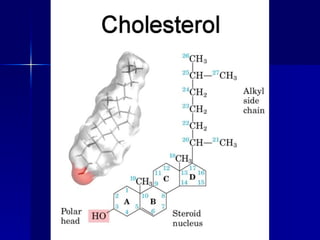
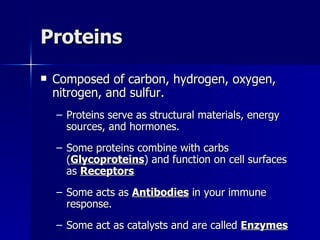
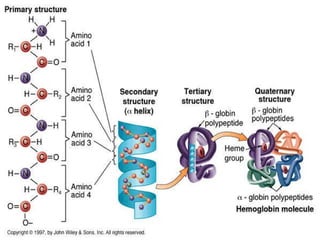
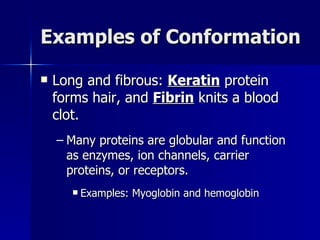
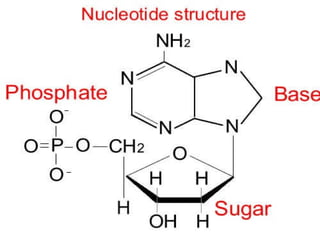
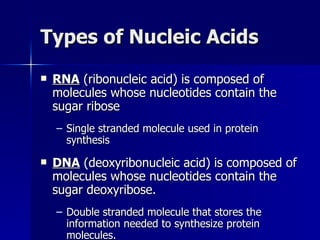
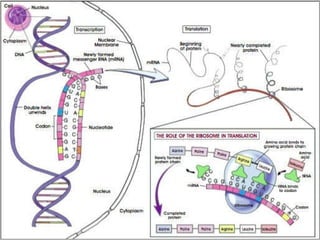
This document summarizes the key chemical constituents of cells. It describes that chemicals in cells can be divided into organic and inorganic substances. The four major inorganic substances are water, oxygen, carbon dioxide, and salts. The four main organic macromolecules that make up living things are carbohydrates, lipids, proteins, and nucleic acids. Each of these molecules has distinct structures and performs important functions for cellular metabolism and heredity.