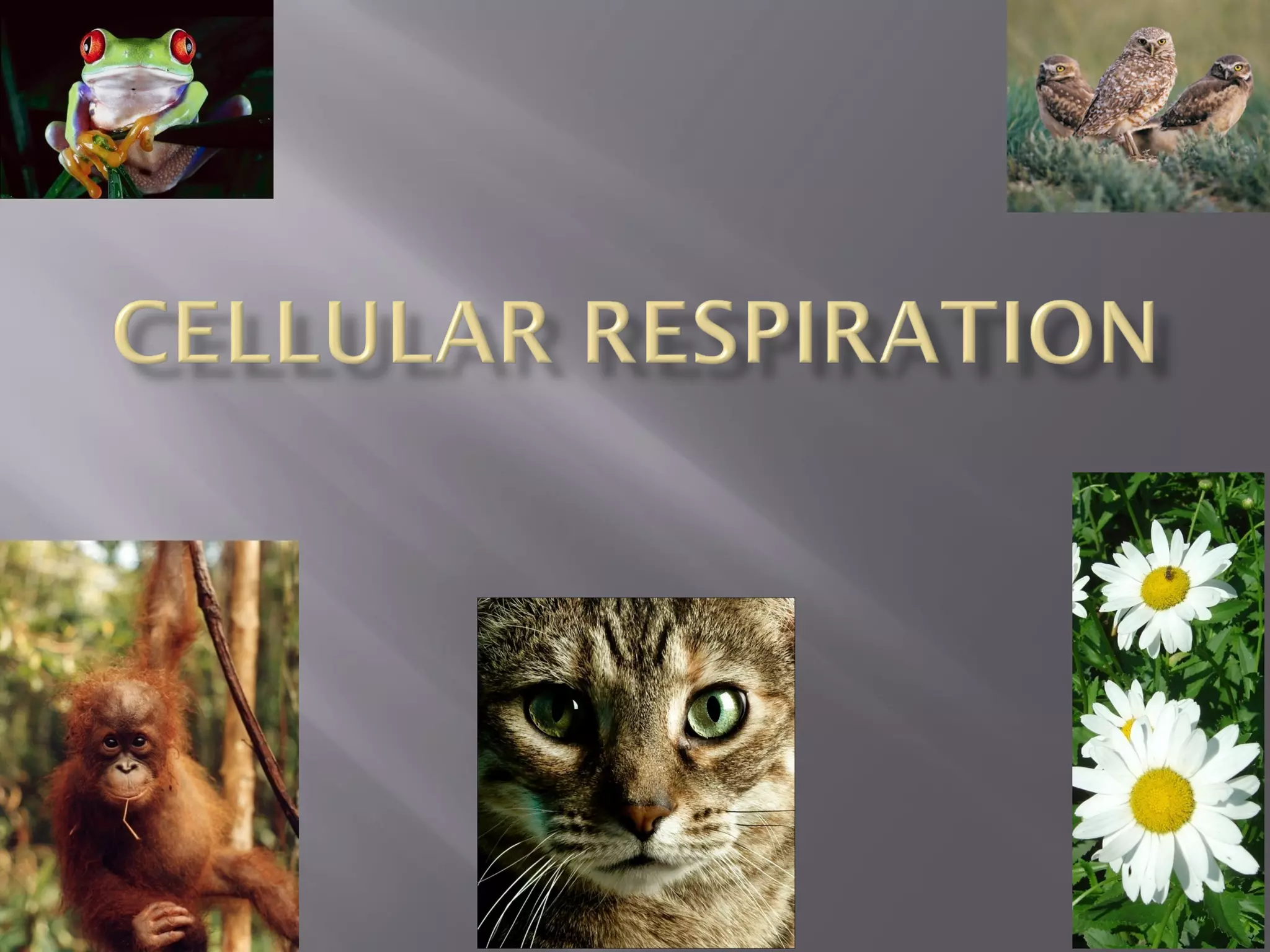
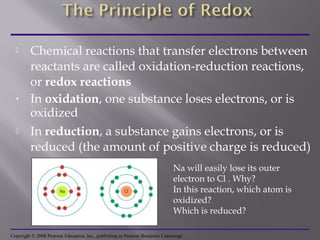
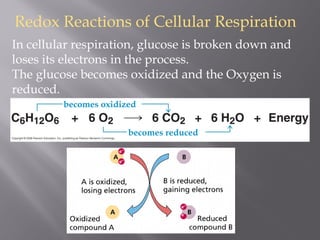
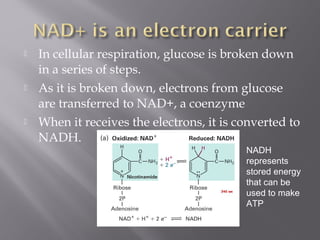
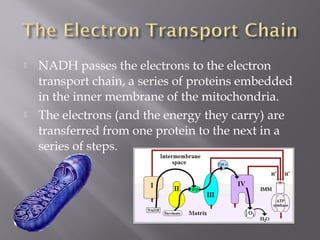
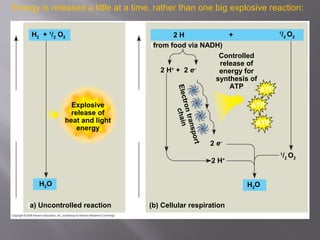
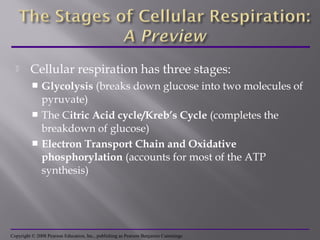
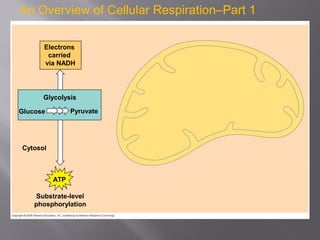
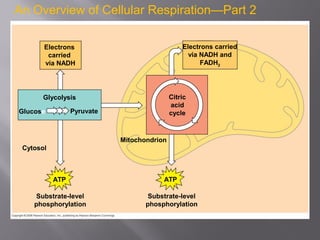
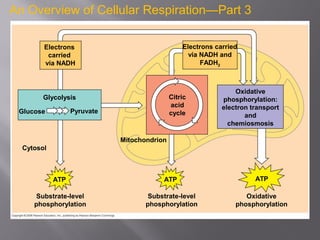
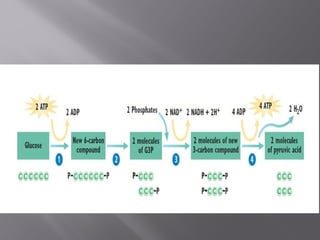
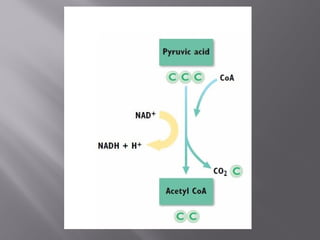
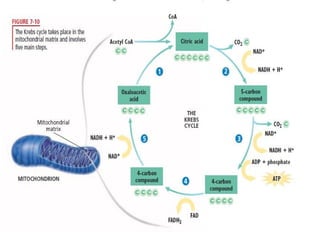
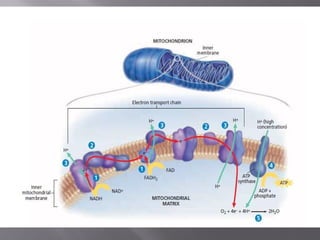
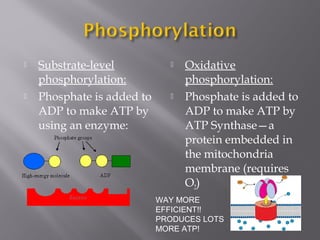
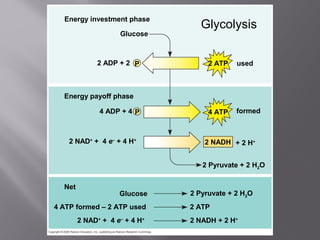
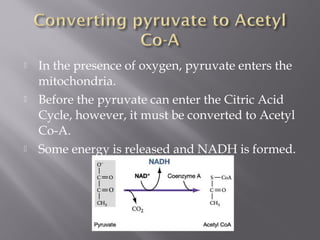
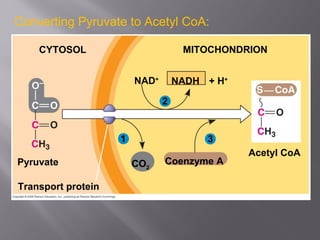
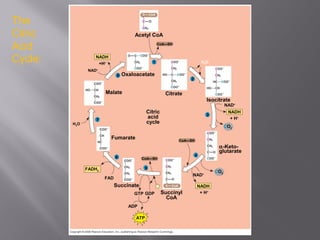
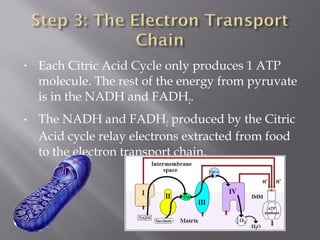
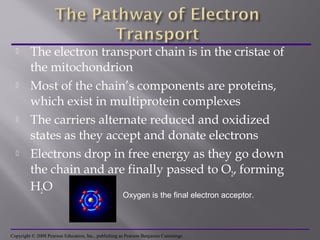
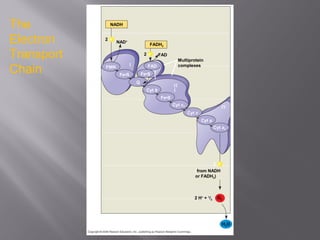
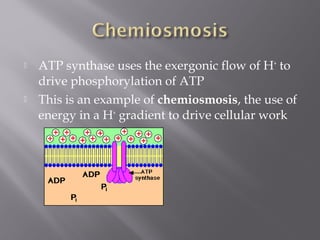
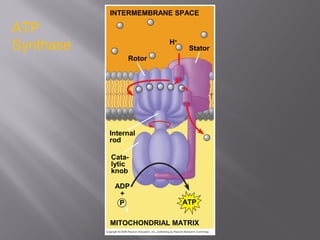
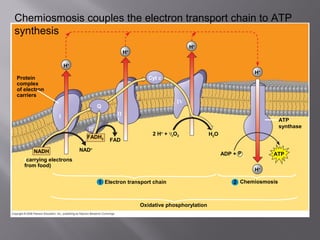
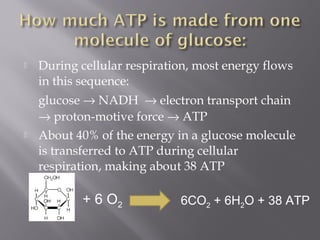
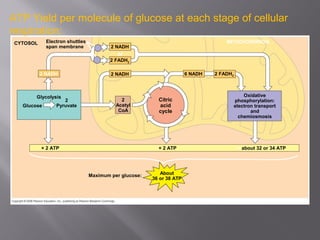
Organisms generate energy through cellular respiration, which has three main stages. In glycolysis, glucose is broken down to pyruvate, producing a small amount of ATP. Pyruvate then enters the citric acid cycle in the mitochondrion, producing more ATP, NADH, and FADH2. These electron carriers supply the electron transport chain, where electrons are transferred to oxygen in a series of redox reactions. This pumps protons out of the mitochondrion, building up a proton gradient that ATP synthase uses to produce the majority of ATP through oxidative phosphorylation.