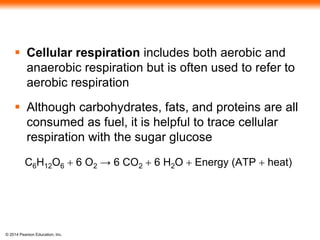
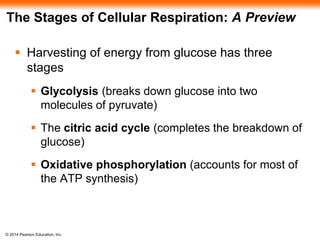
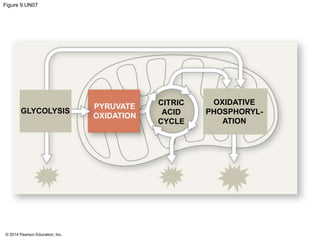
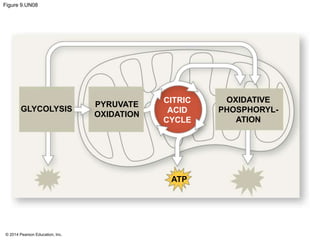
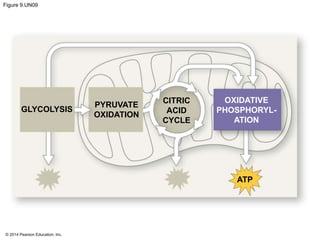
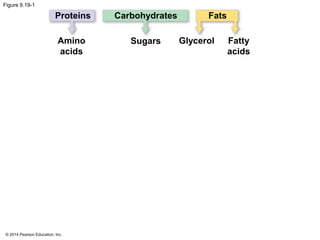
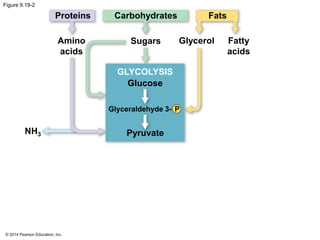
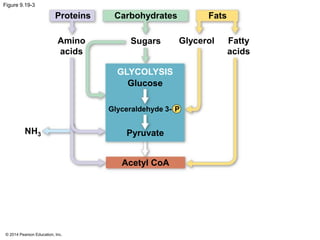
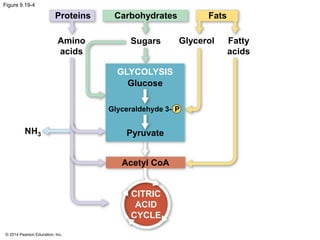
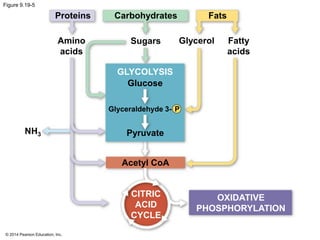
1. The document outlines a lecture presentation on cellular respiration and fermentation. It discusses the three stages of cellular respiration - glycolysis, the citric acid cycle, and oxidative phosphorylation.
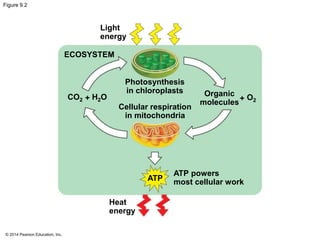
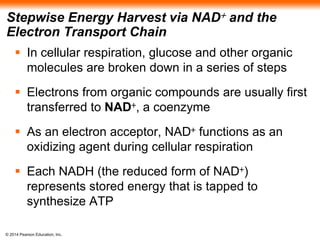
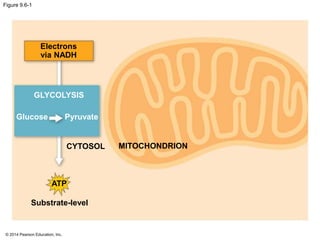
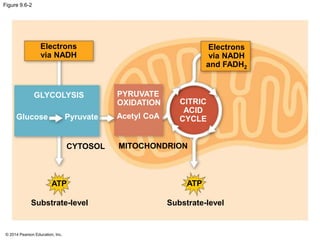
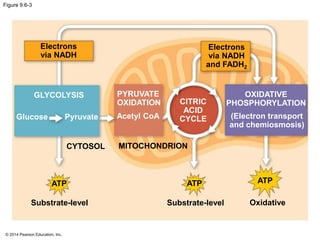
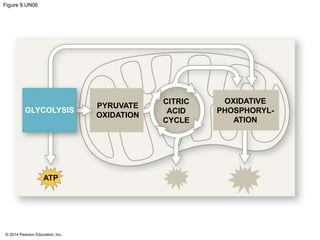
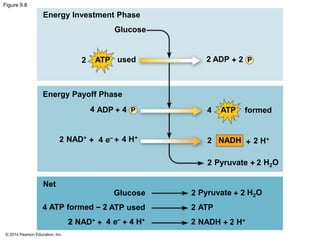
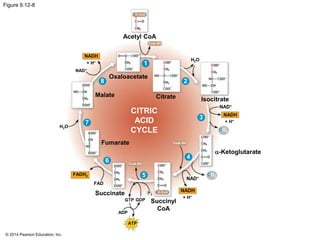
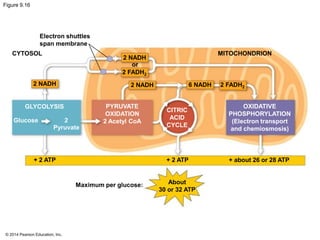
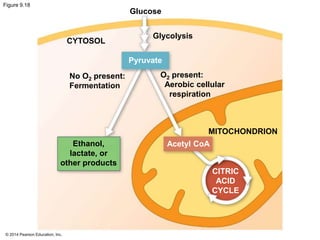
2. Glycolysis breaks down glucose into pyruvate and generates a small amount of ATP. The citric acid cycle then completes the oxidation of pyruvate and generates more ATP.
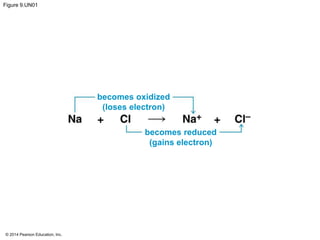
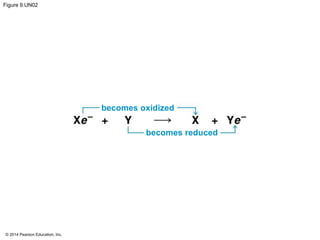
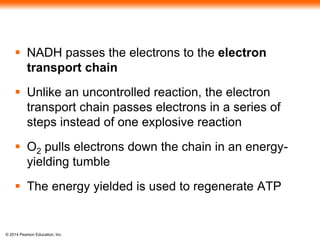
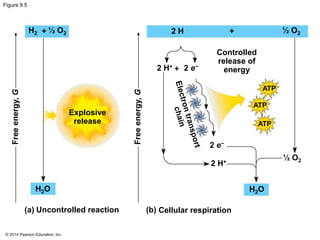
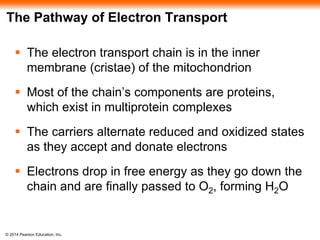
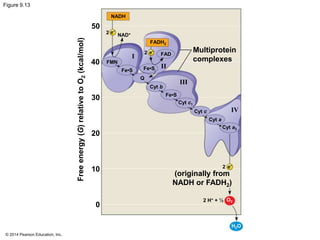
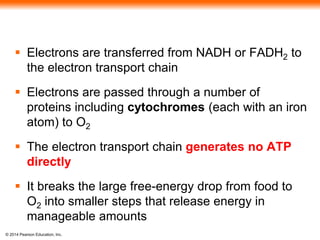
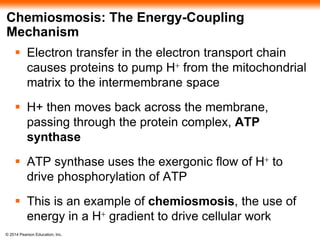
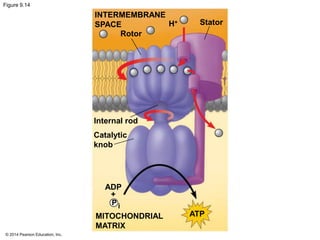
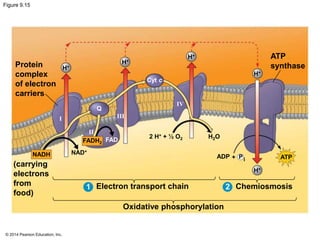
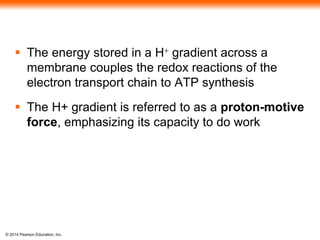
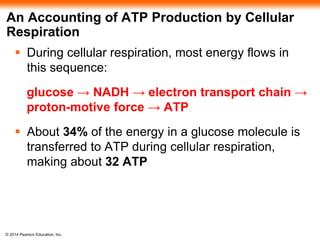
3. Oxidative phosphorylation, powered by redox reactions, generates the majority of ATP through chemiosmosis and the electron transport chain in the mitochondria. This three-stage process extracts energy from glucose and other organic molecules to produce ATP.