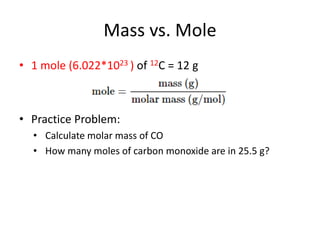
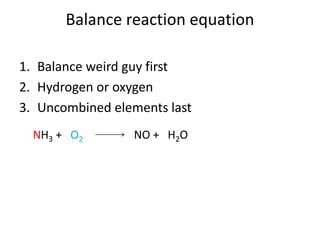
This document provides an outline for a general chemistry course lecture on stoichiometry. It introduces key concepts like atomic mass units, moles, molar mass, reaction stoichiometry, limiting and excess reactants. Example problems are provided to illustrate how to calculate the number of atoms or moles of a substance, balance chemical equations, determine limiting reagents, and use stoichiometry to quantify reactants and products in a chemical reaction.