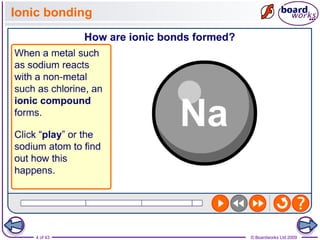
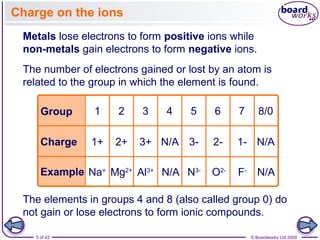
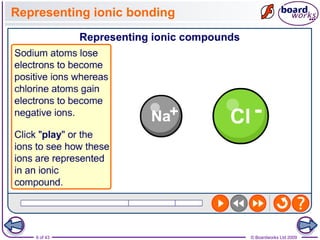
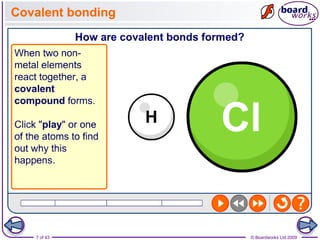
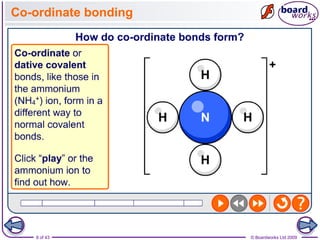
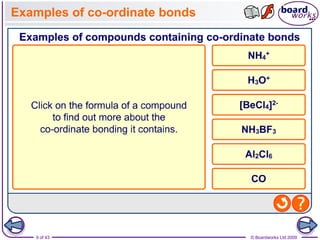
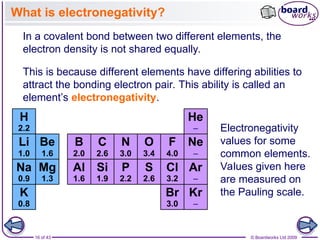
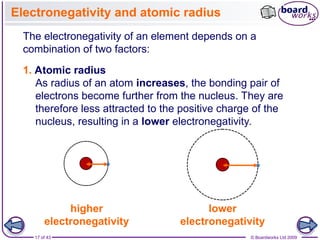
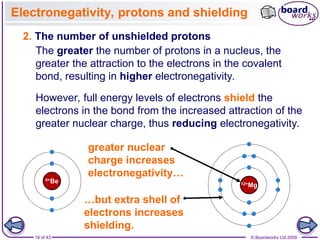
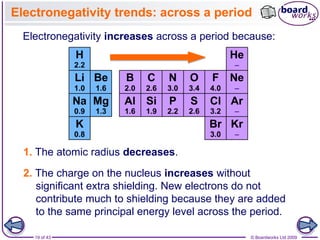
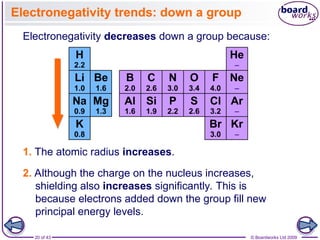
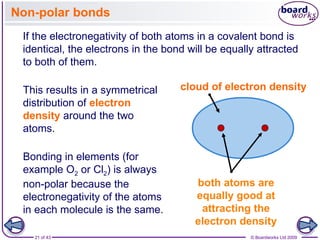
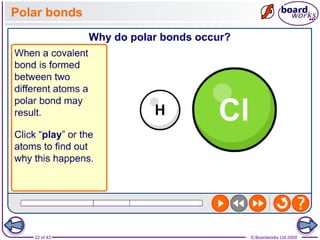
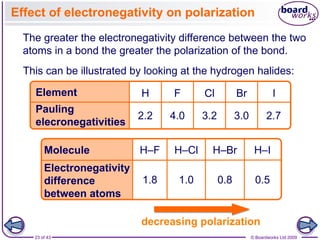
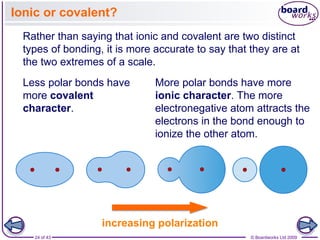
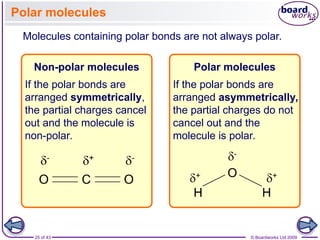
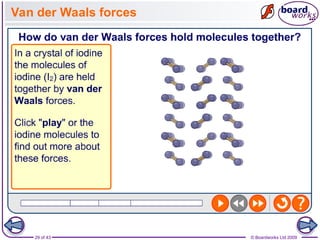
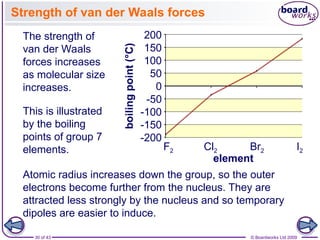
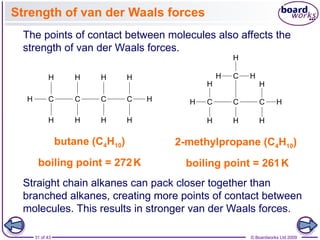
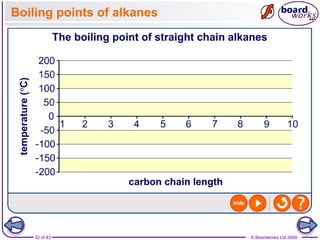
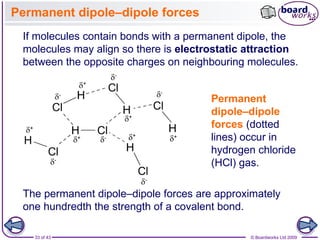
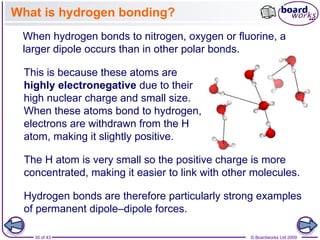
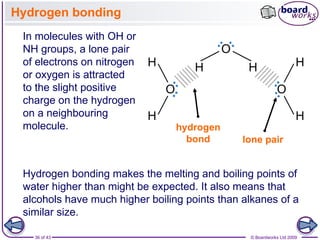
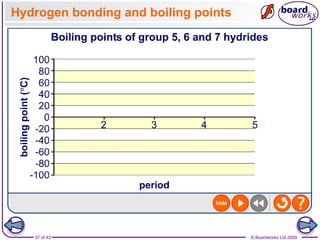
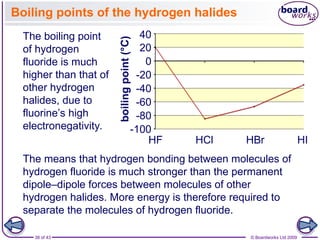
The document discusses three main types of chemical bonding: ionic, covalent, and metallic, explaining their formation and characteristics. It also covers the concept of electronegativity and its impact on bond polarity, along with intermolecular forces including hydrogen bonds, permanent dipole-dipole forces, and van der Waals forces. Additionally, it highlights the factors that influence the strength of these bonds and forces, including ion charge, atomic size, and molecular shape.