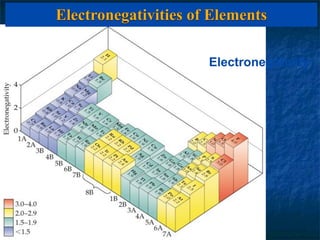
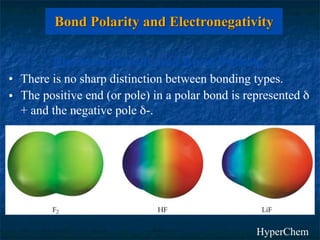
The electronegativity of an element is a measure of how strongly it attracts electrons in a covalent bond. Electronegativity increases left to right and top to bottom in a period, and metals have the lowest values while nonmetals have the highest. The difference in electronegativity between two bonded atoms indicates bond type - ionic bonds form when difference is >1.7, covalent bonds form when difference is <1.7, and polar covalent bonds form for differences in between. Electronegativity values can be used to predict and investigate bond types.