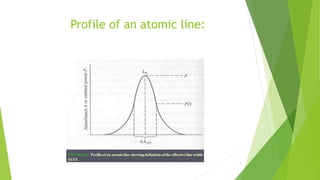
The document presents an overview of atomic line width and the uncertainty principle, detailing the definition, significance, and sources of line broadening that affect atomic absorption and emission spectra. It highlights that narrow lines are crucial for reducing interference in spectroscopy and discusses various factors like the uncertainty effect and the Doppler effect that contribute to line broadening. Ultimately, it underscores the importance of understanding these concepts for the design of instruments used in atomic emission spectroscopy.