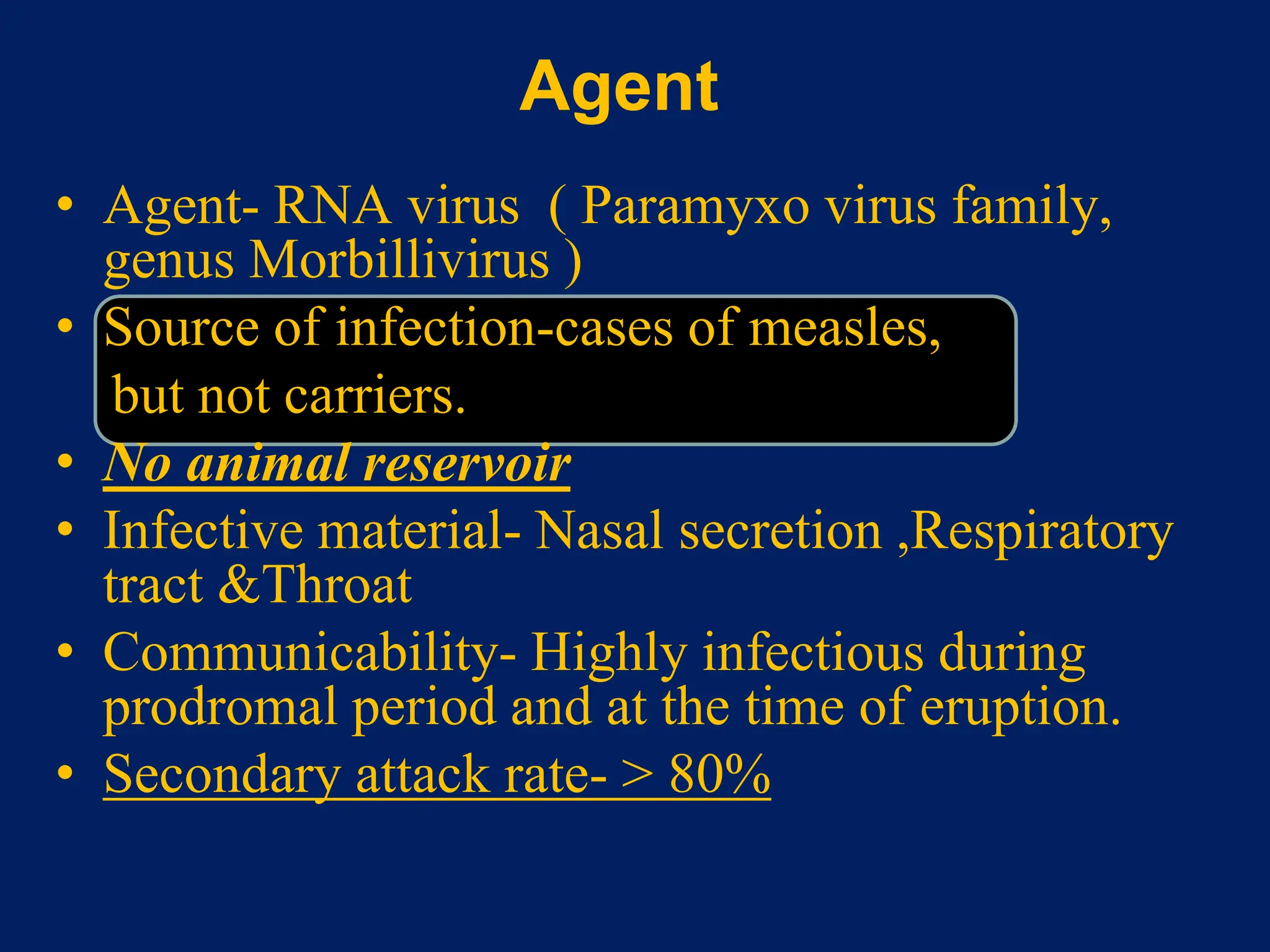
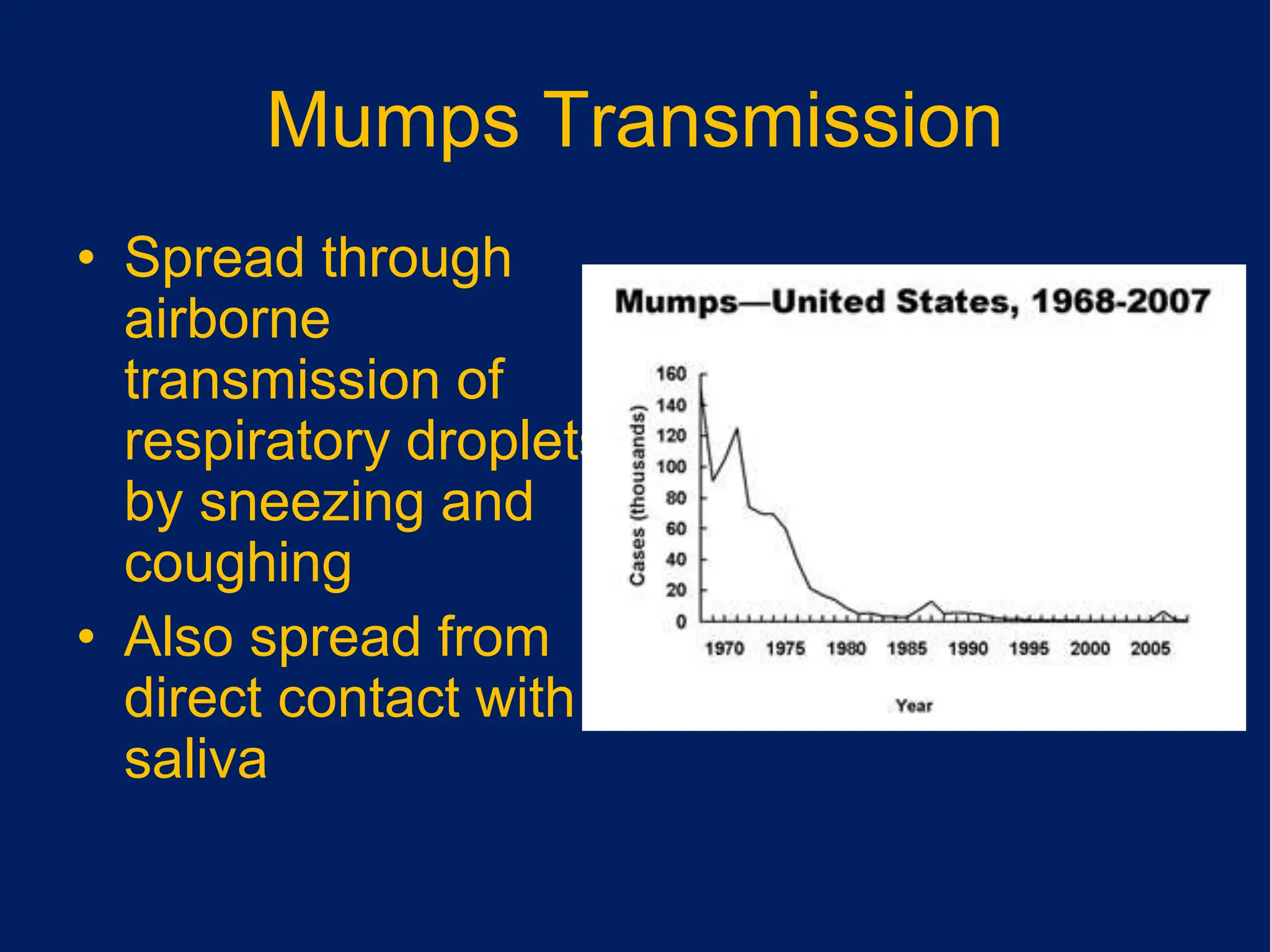
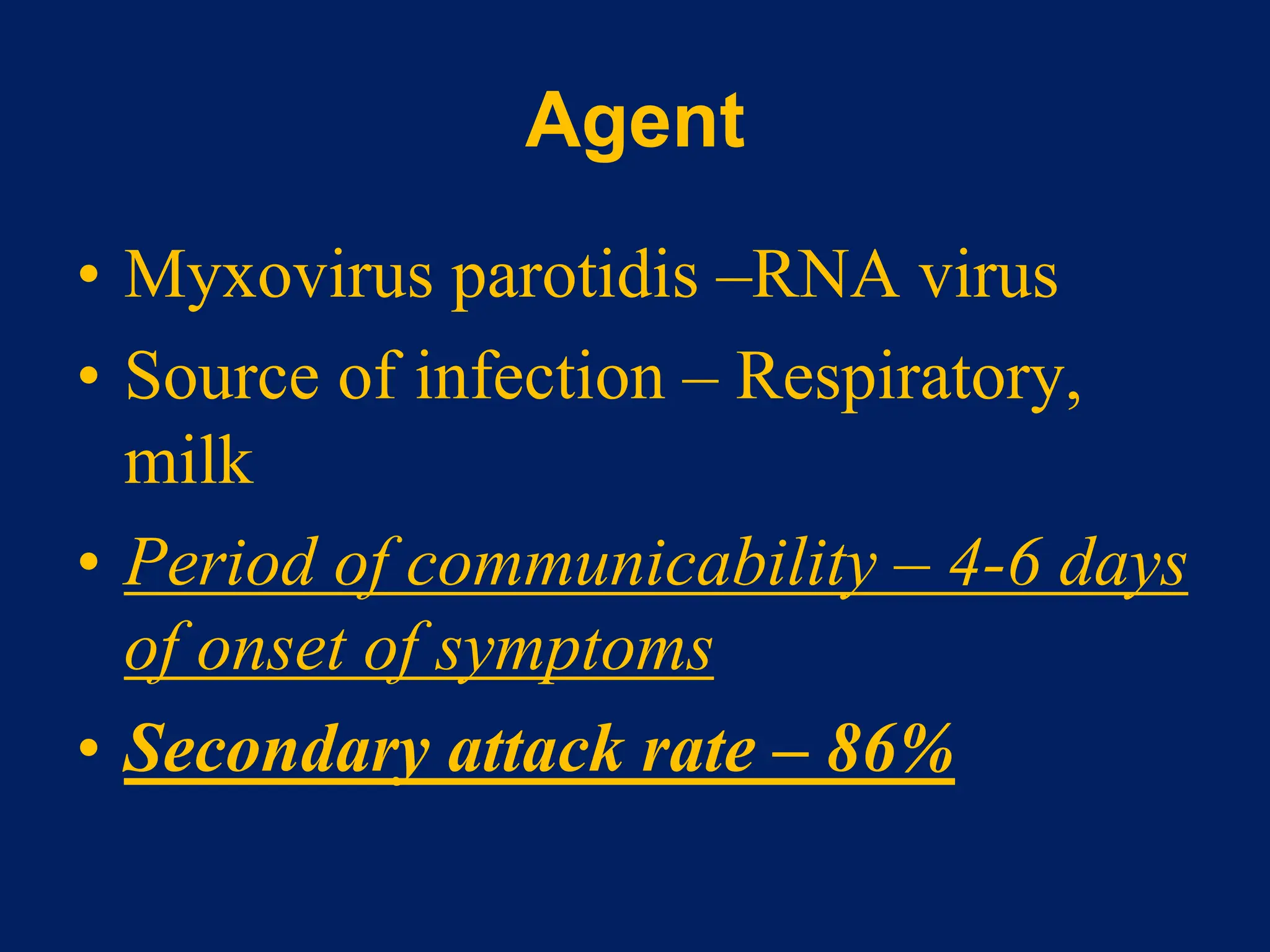
- Measles, mumps, and rubella are caused by viruses. Measles is caused by a paramyxovirus, mumps by a myxovirus, and rubella by a rubivirus.
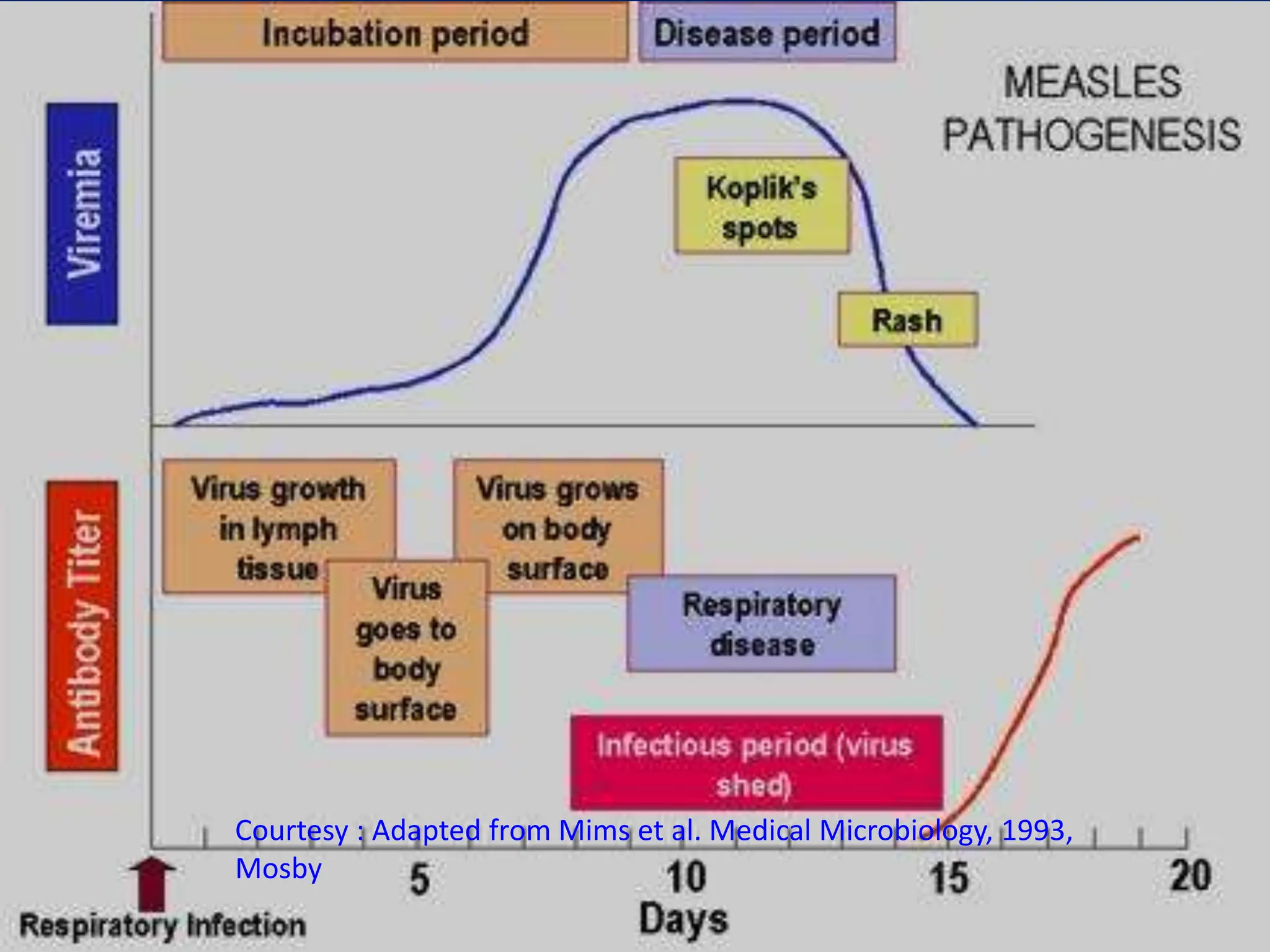
- They are spread through respiratory droplets. Measles and mumps have an incubation period of 7-18 days, while rubella is 14-21 days.
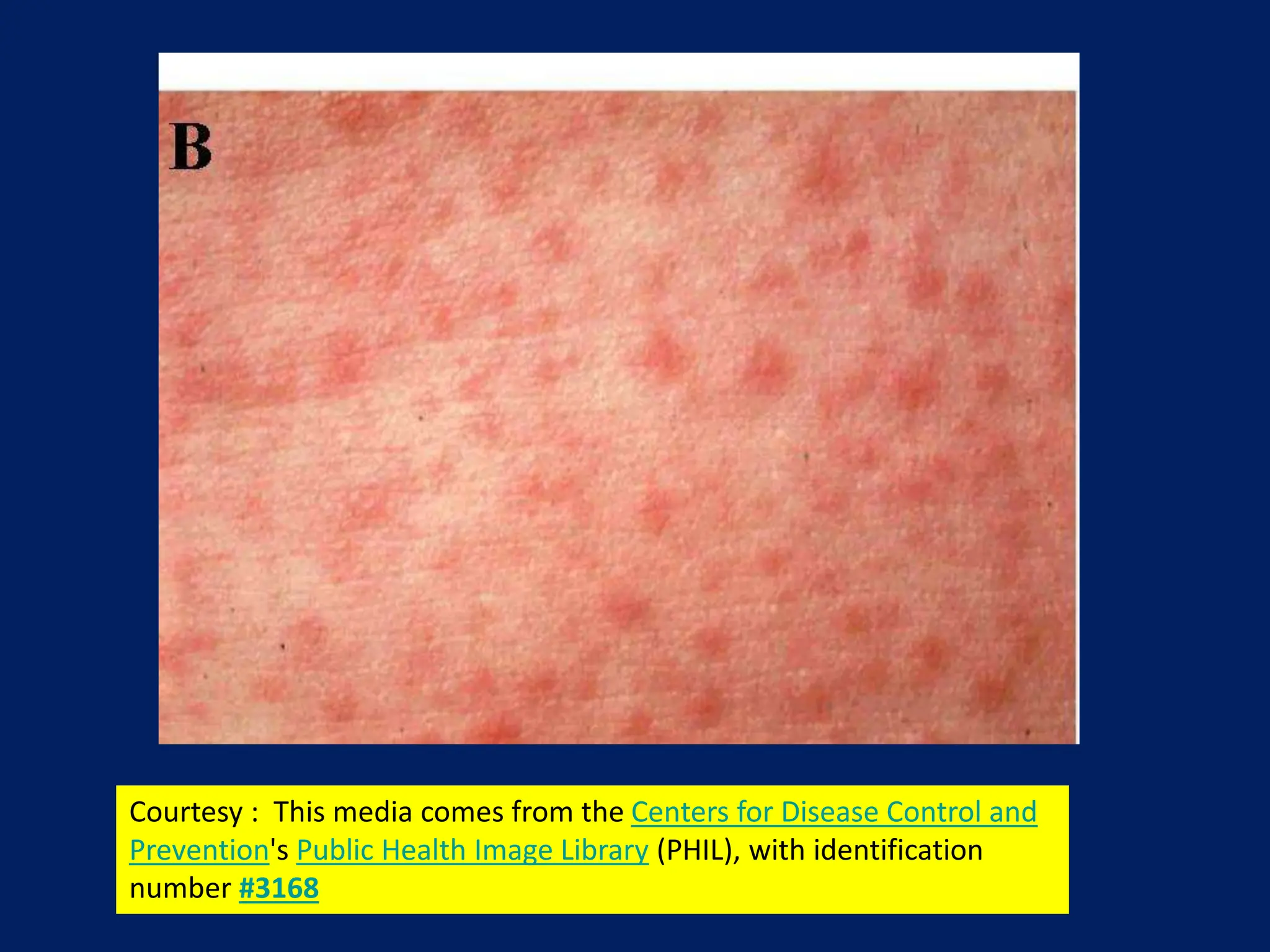
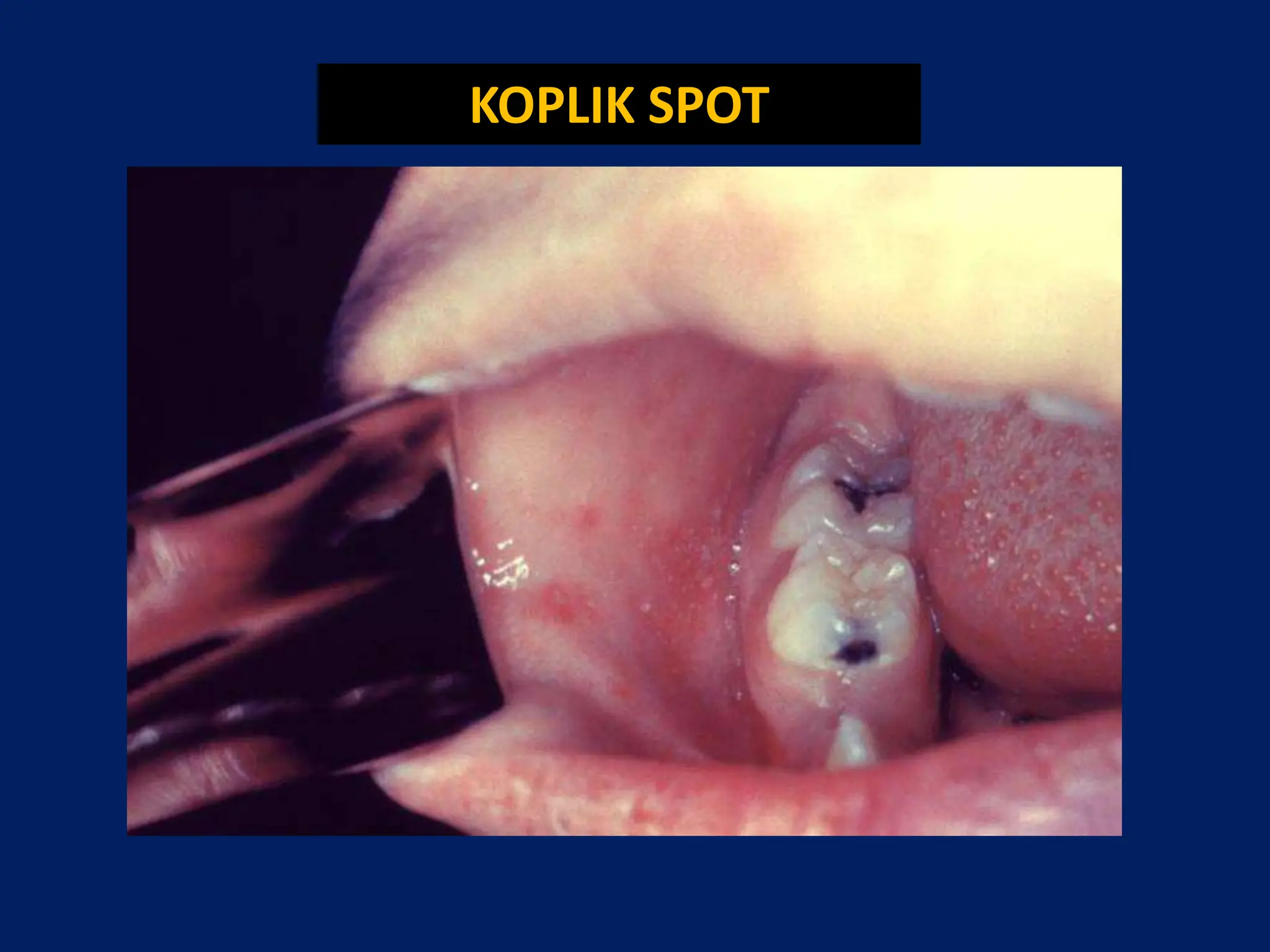
- Common symptoms include fever, rash, and lymphadenopathy. Complications can include pneumonia, encephalitis and congenital rubella syndrome.
- Vaccines provide effective protection. MMR vaccine contains live attenuated viruses and is recommended in 2 doses for children.