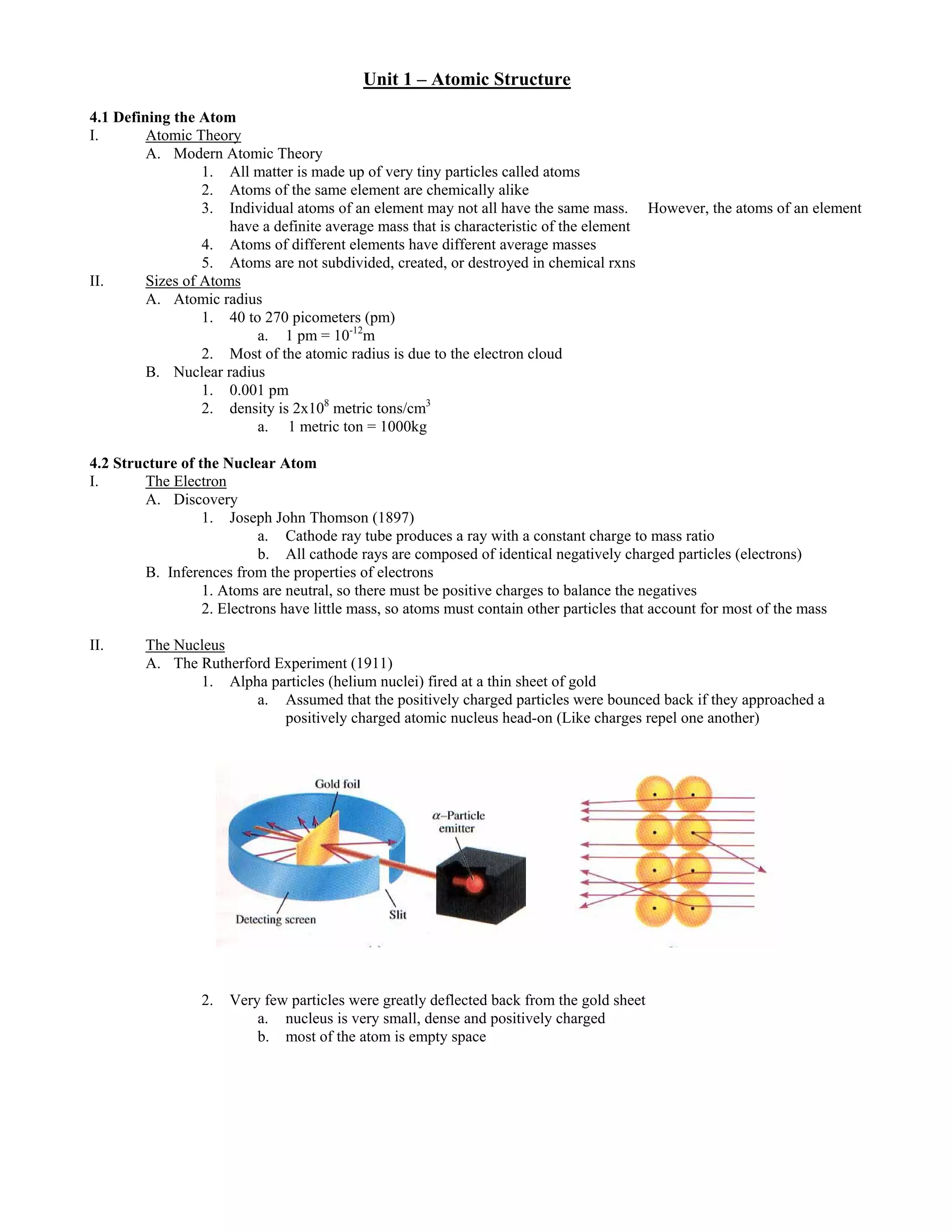
This document provides an overview of atomic structure and nuclear chemistry concepts. It defines the atom and its components, including electrons, protons, and neutrons. It describes atomic models including Thomson's discovery of the electron and Rutherford's nuclear model. Key concepts are atomic number, mass number, isotopes, radioactive decay via alpha, beta, and gamma emission. Nuclear reactions like fission and fusion are summarized. Radiation types are compared by their penetrating abilities.