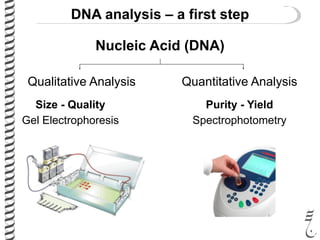
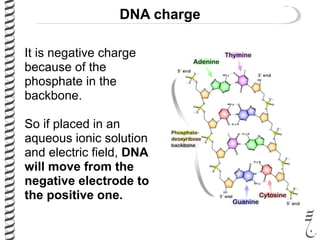
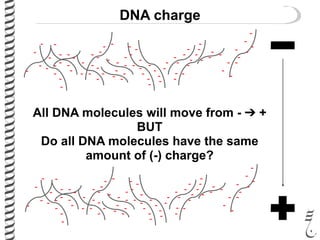
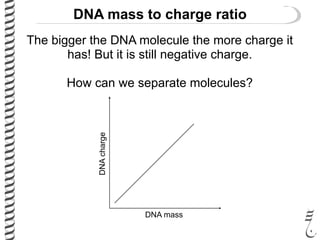
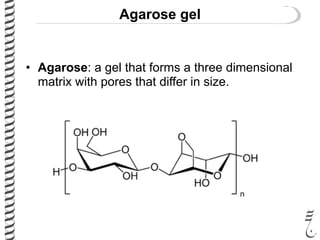
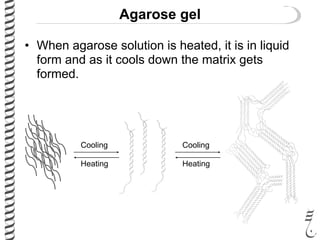
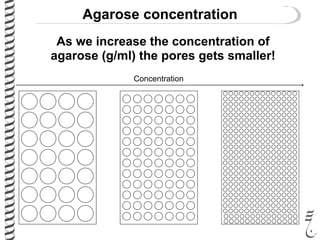
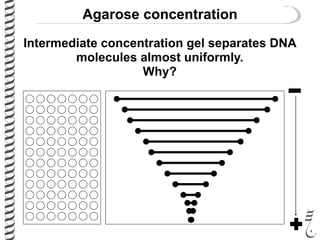
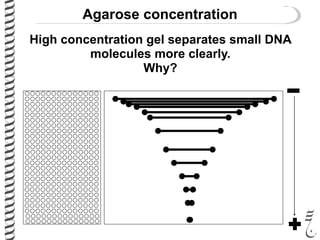
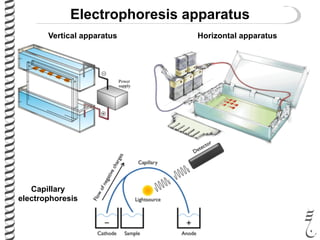
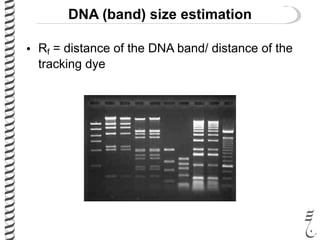
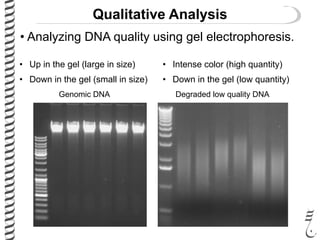
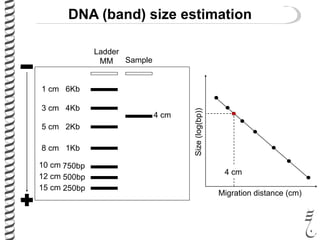
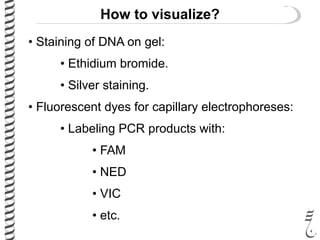
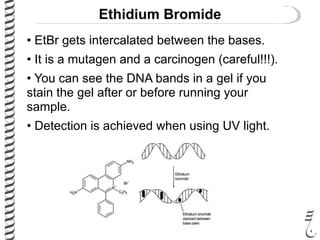
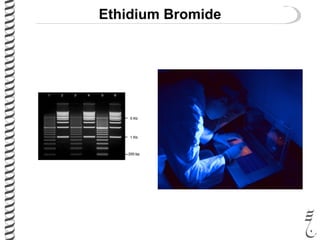
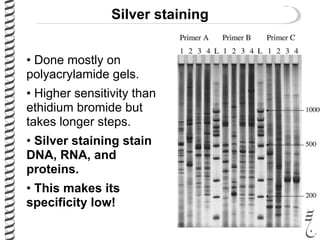
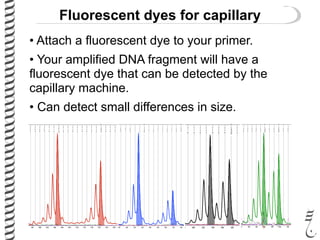
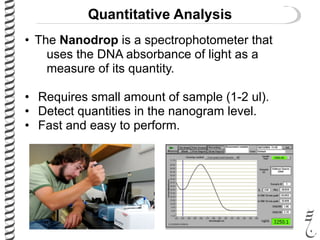
This document summarizes techniques for analyzing DNA quality and quantity. It discusses running DNA samples on a gel to qualitatively assess size and quality, and using a spectrophotometer to quantitatively measure DNA amount and purity. Specifically, it covers how DNA moves in a gel based on its charge, how agarose concentration affects separation, staining DNA with ethidium bromide, using fluorescent dyes in capillary electrophoresis, and quantifying DNA with a spectrophotometer or Nanodrop. The goal is to learn fundamental methods for characterizing DNA samples before further analysis.