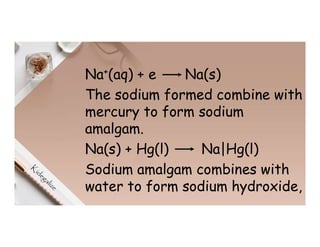
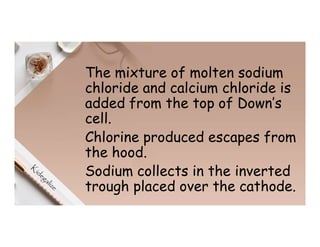
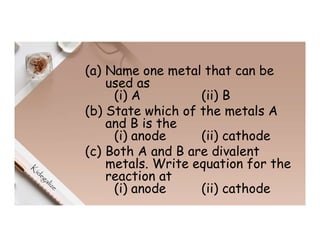
1. Electricity can affect substances in different ways depending on whether they are conductors, insulators, electrolytes or non-electrolytes. Conductors allow electric current to pass through due to mobile electrons, while insulators do not due to electrons locked in bonds.
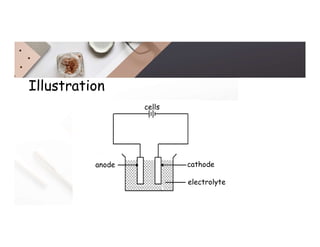
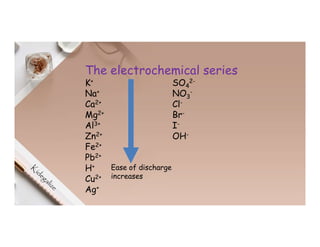
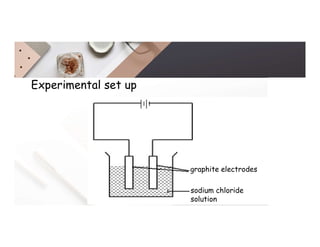
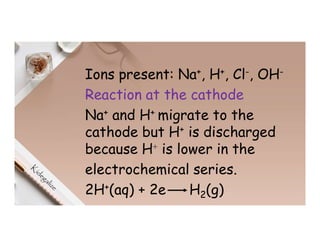
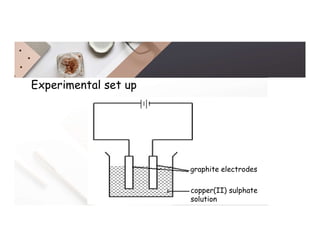
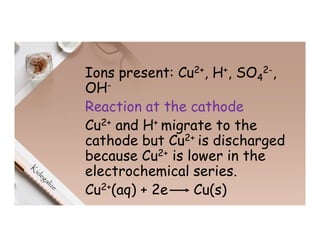
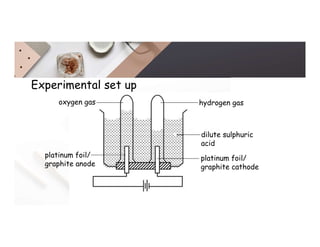
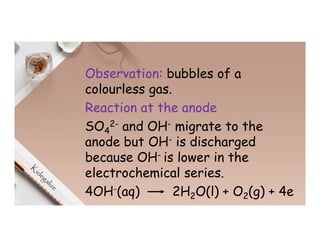
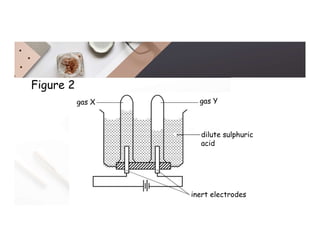
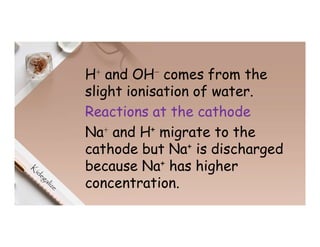
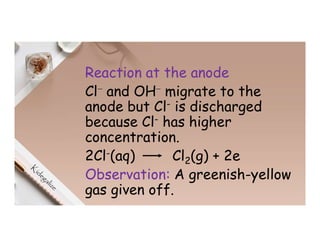
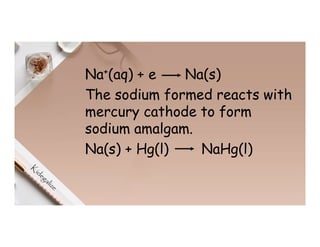
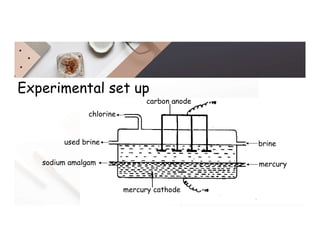
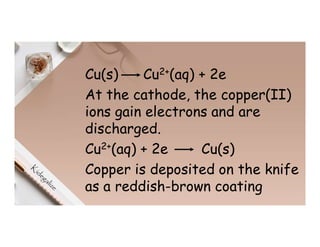
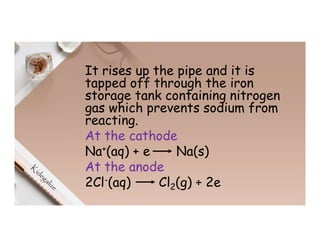
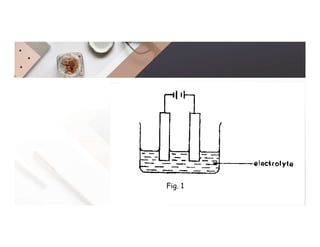
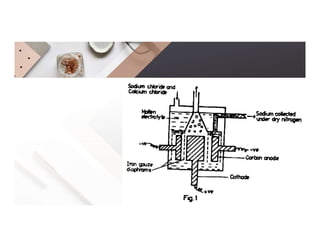
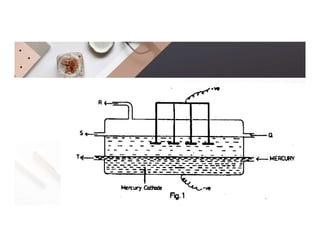
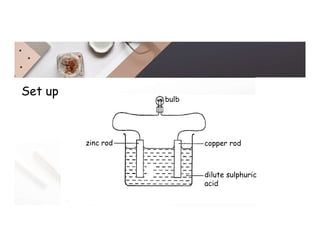
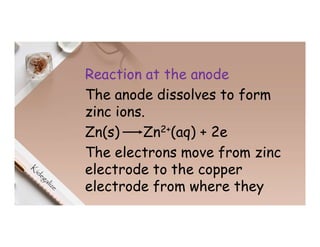
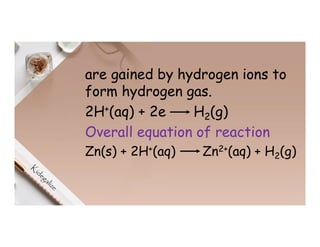
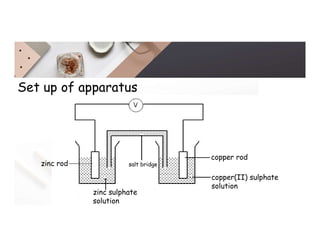
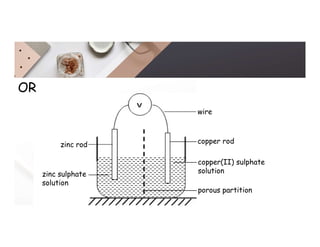
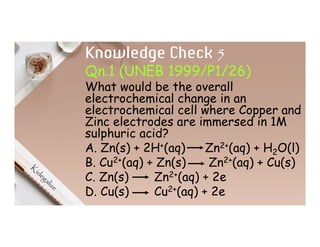
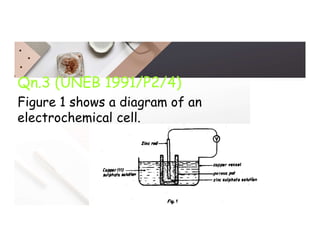
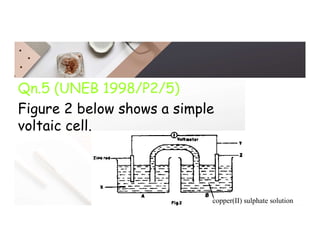
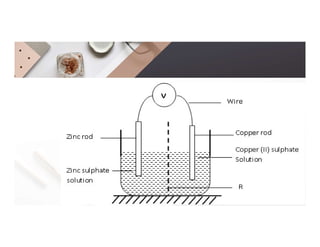
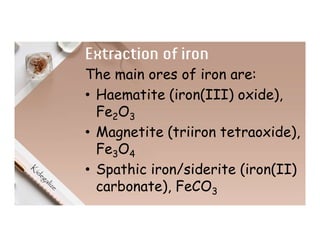
2. Electrolysis is the decomposition of an electrolyte solution or melt by passing an electric current through it. At the anode, oxidation occurs as ions lose electrons. At the cathode, reduction occurs as ions gain electrons. Common electrolytes decomposed include NaCl, CuSO4 and acidified water.
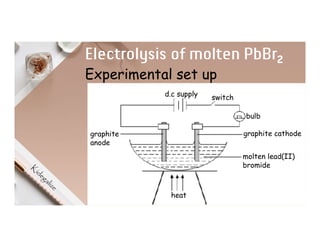
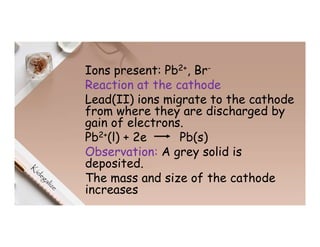
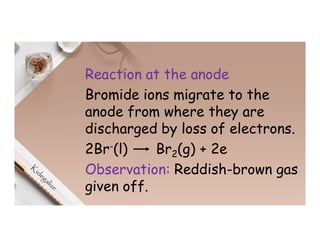
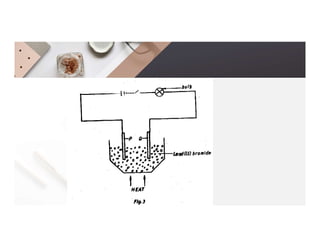
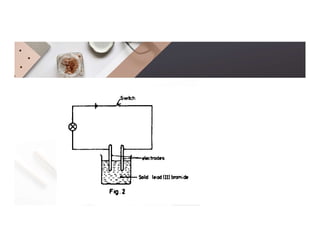
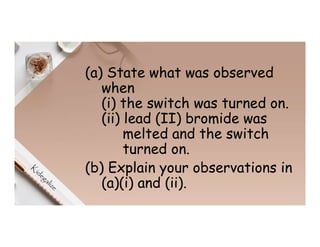
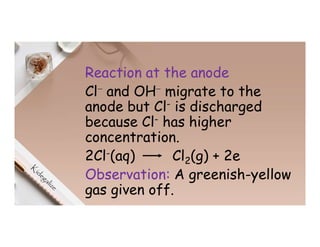
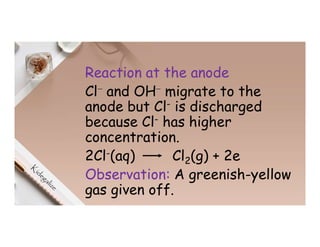
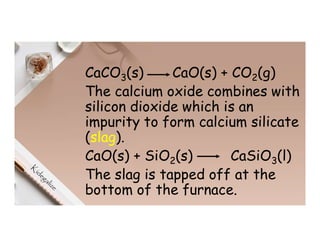
3. During the electrolysis of molten lead(II) bromide, lead metal deposits at the cathode while bromine