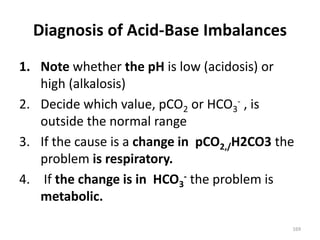
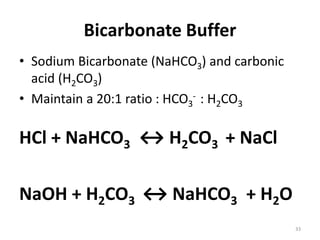
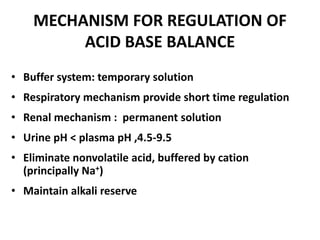
This document discusses acid-base balance and the mechanisms that regulate blood pH homeostasis. It begins by defining pH and explaining why blood pH is tightly regulated within a narrow range. It then describes the various sources of acids and bases in the body from metabolic processes. The three main mechanisms that regulate blood pH are: 1) blood buffer systems that rapidly neutralize added acids or bases, 2) respiratory control that exhales volatile acids, and 3) renal control through bicarbonate reabsorption and acid excretion over hours. Imbalances can occur if these mechanisms fail, resulting in acidosis if pH decreases below 7.35 or alkalosis if it increases above 7.45.



![What Is pH?
• pH is a Hydrogen ion concentration.
• pH = - log [H+]
• Different compartment of human
body has specific pH.
• pH has role in Enzyme activity.](https://image.slidesharecdn.com/finalacidbasebalane1-221117060020-ac3dc66a/85/acid_base_balane-1-pptx-4-320.jpg)























































































































































![Anion Gap Acidosis:
• Anion gap >12 mmol/L; caused by a decrease
in [HCO3 -]
• Balanced by an increase in an unmeasured
acid ion from either endogenous production
or exogenous ingestion (normochloremic
acidosis).](https://image.slidesharecdn.com/finalacidbasebalane1-221117060020-ac3dc66a/85/acid_base_balane-1-pptx-161-320.jpg)


![Henderson Hasselbalch Equation
• pH= pka +log [HCO3-]/[H2CO3]
• At pH 7.4 the ratio of HCO3-/H2CO3
is 1:20.
• A buffer is most effective when
pH=pKa
• When concentration of salt and acid
are equal.](https://image.slidesharecdn.com/finalacidbasebalane1-221117060020-ac3dc66a/85/acid_base_balane-1-pptx-164-320.jpg)