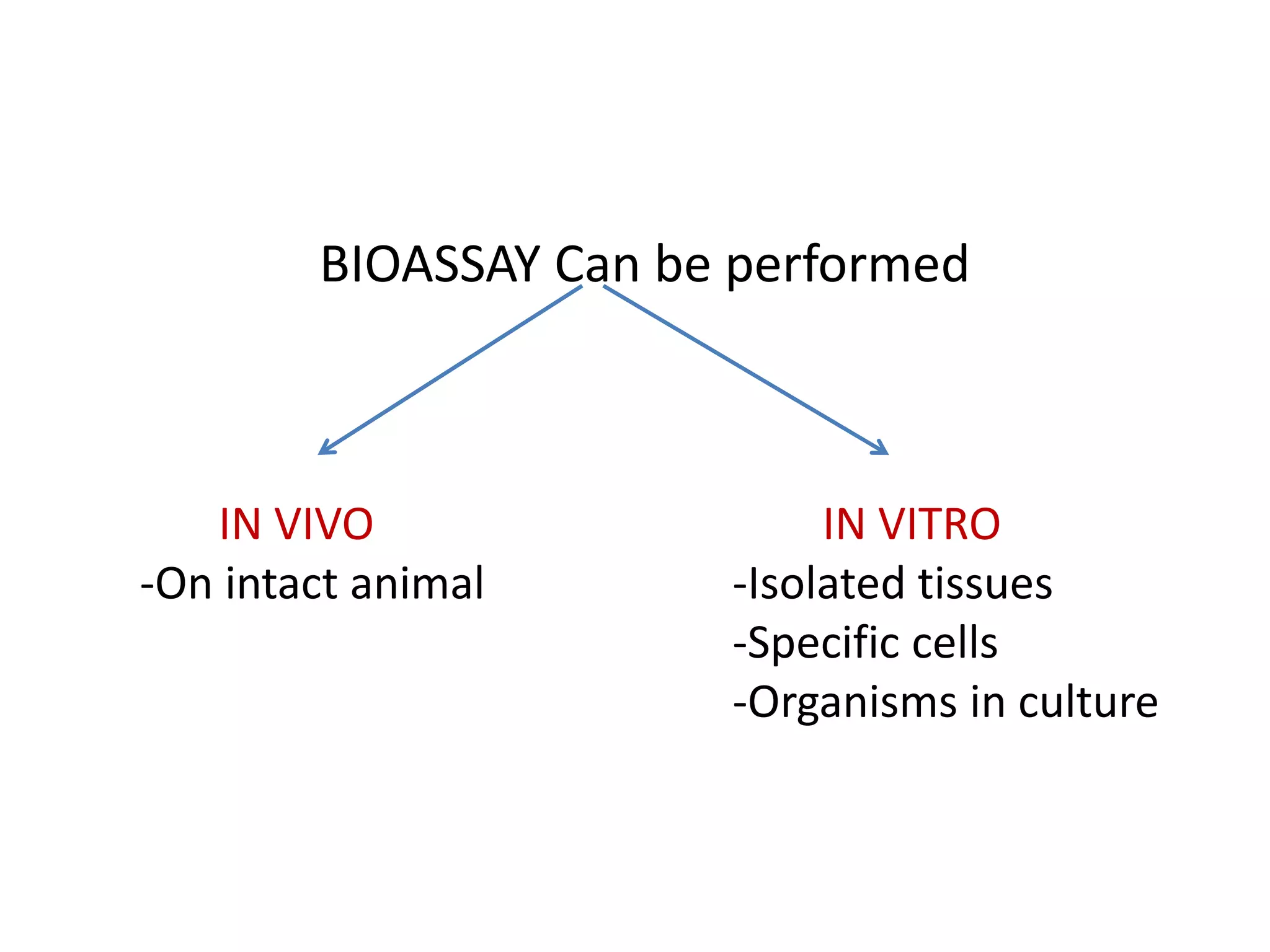
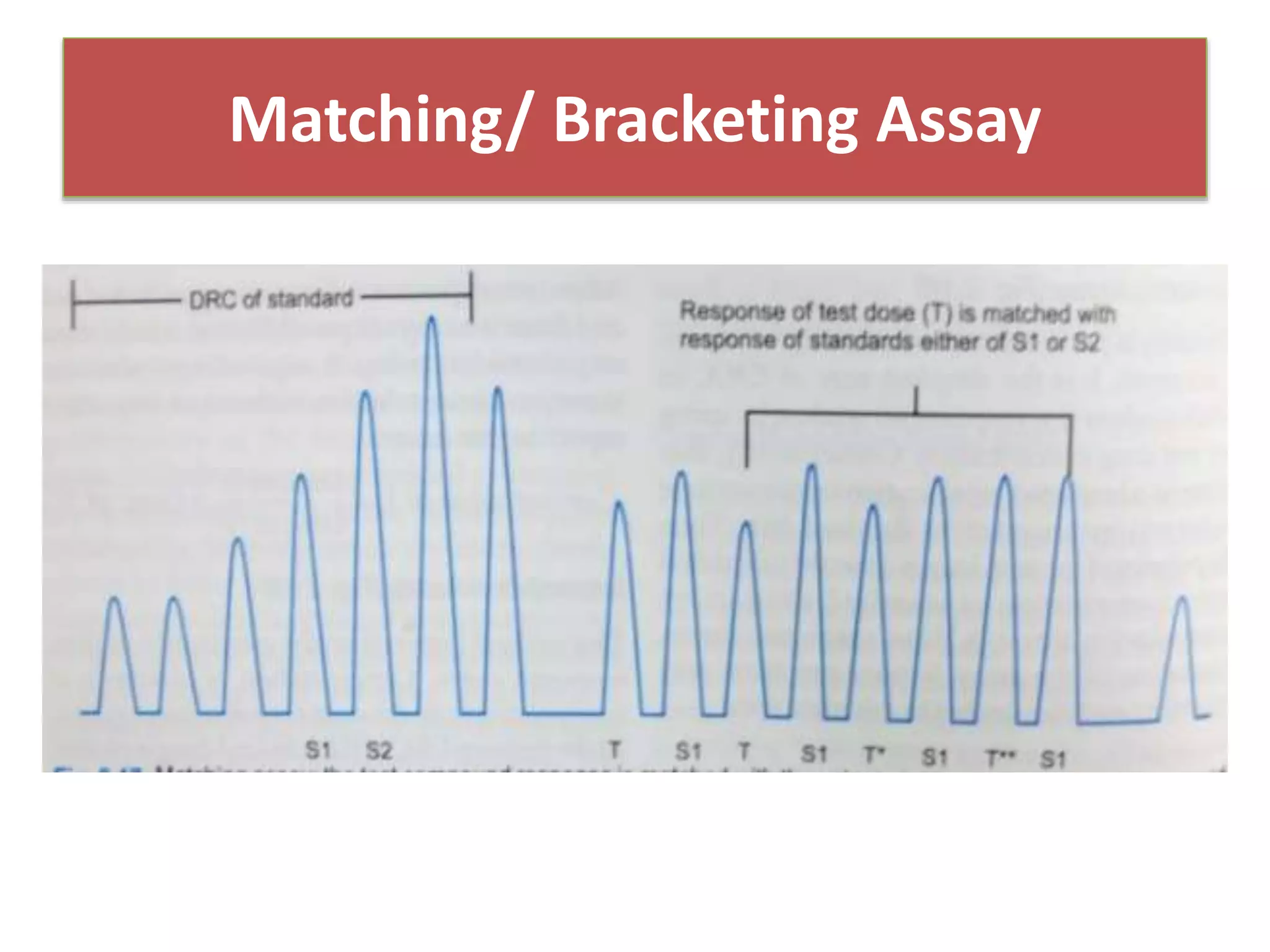
The document discusses bioassays and dose-response curves, detailing methods to measure the presence and potency of drugs using various biological and chemical assays. It outlines principles, types of assays, and the detailed procedures for conducting bioassays, emphasizing factors like sensitivity, specificity, and reproducibility. The content also includes information on the assembly of instruments used in bioassays, along with theoretical calculations for determining drug potency and effectiveness.