The presentation summarizes the key aspects of bioassay including:
1) Bioassay is defined as the estimation of the potency of an active principle in a preparation using biological methods and living organisms or tissues.
2) The main types of bioassays are graded response assays which measure proportional responses, and quantal assays which measure all-or-none thresholds.
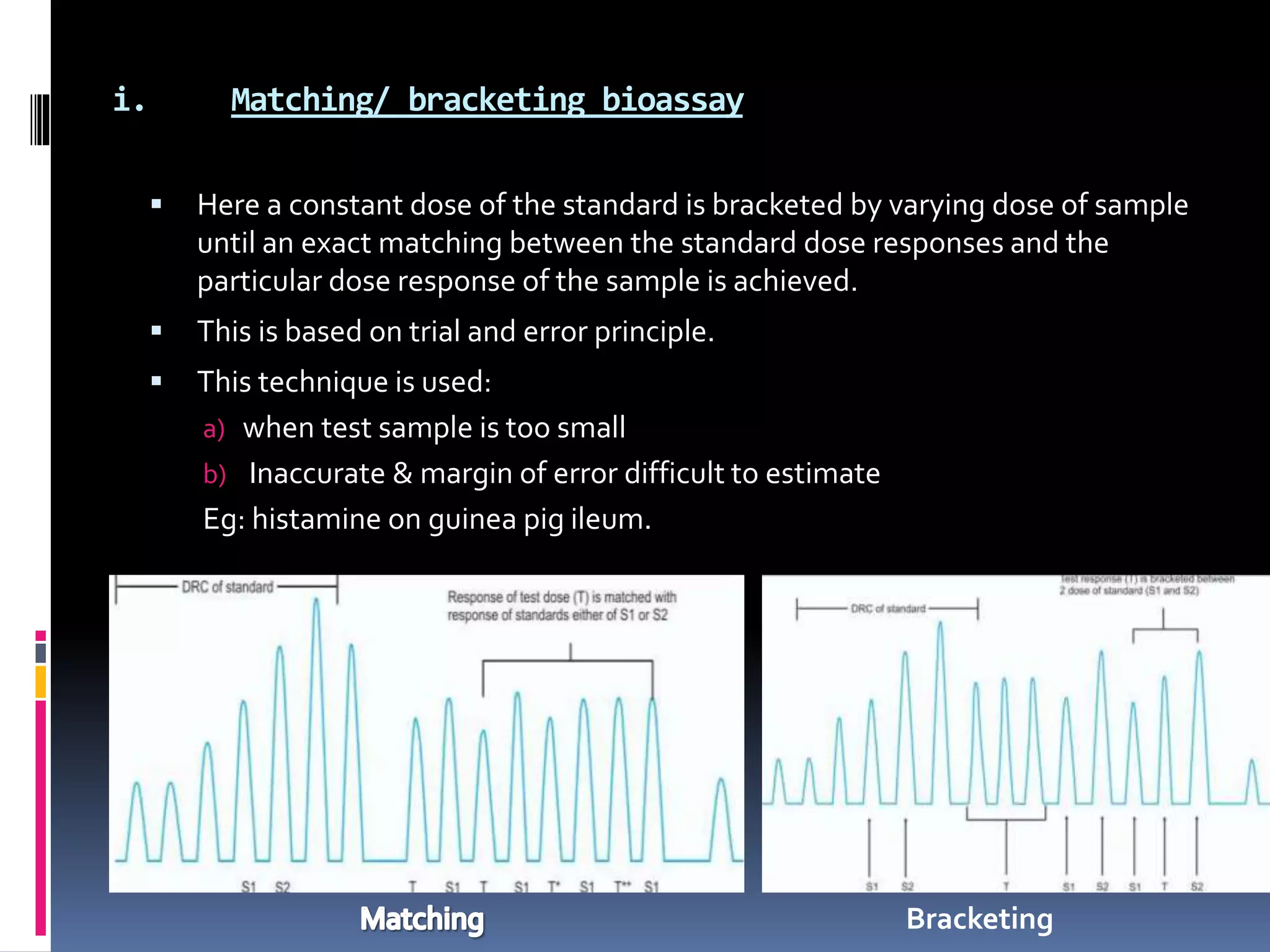
3) Graded bioassays include interpolation, matching, and multiple point assays while common quantal assays use lethal dose 50 (LD50) determinations.