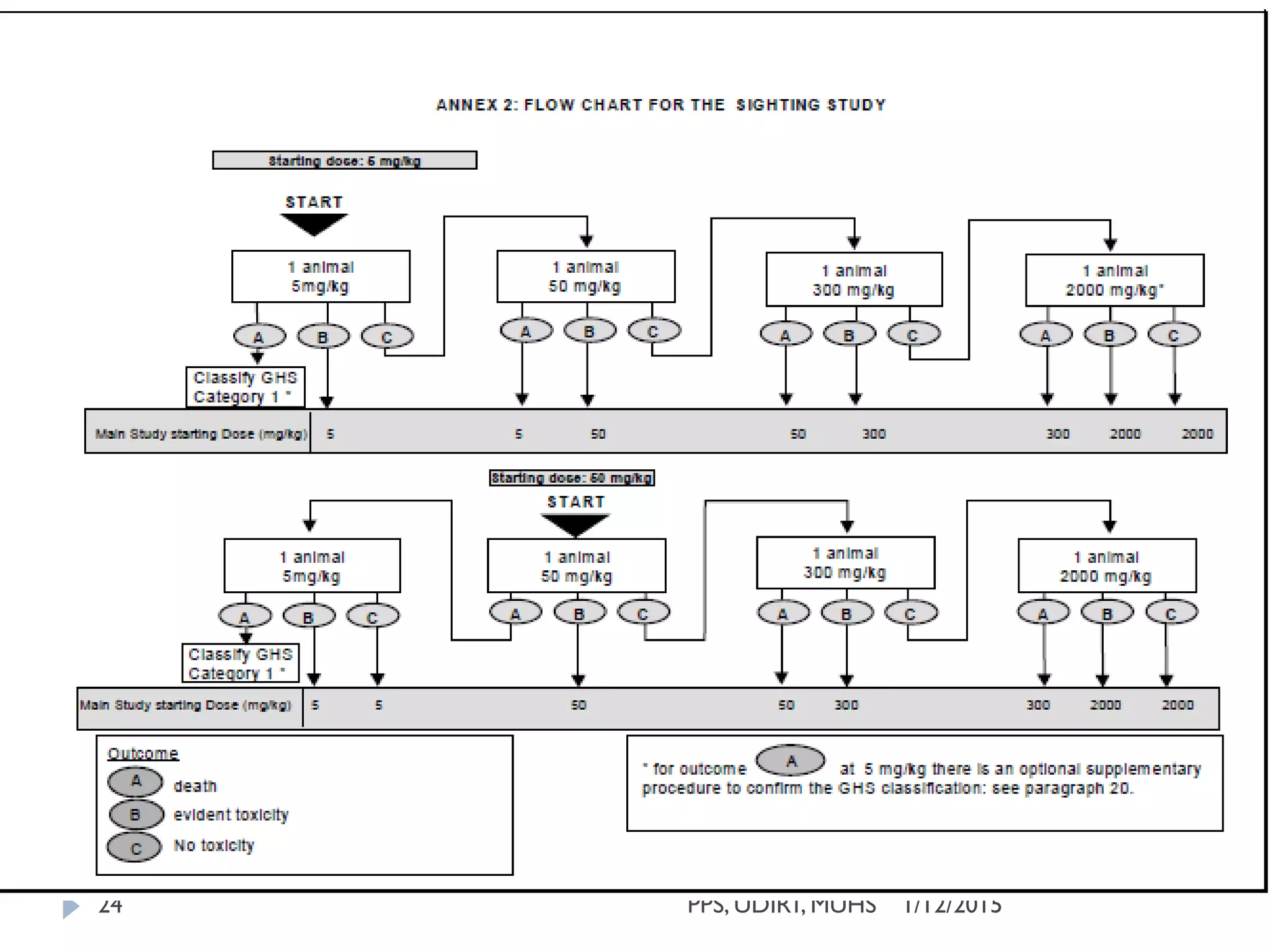
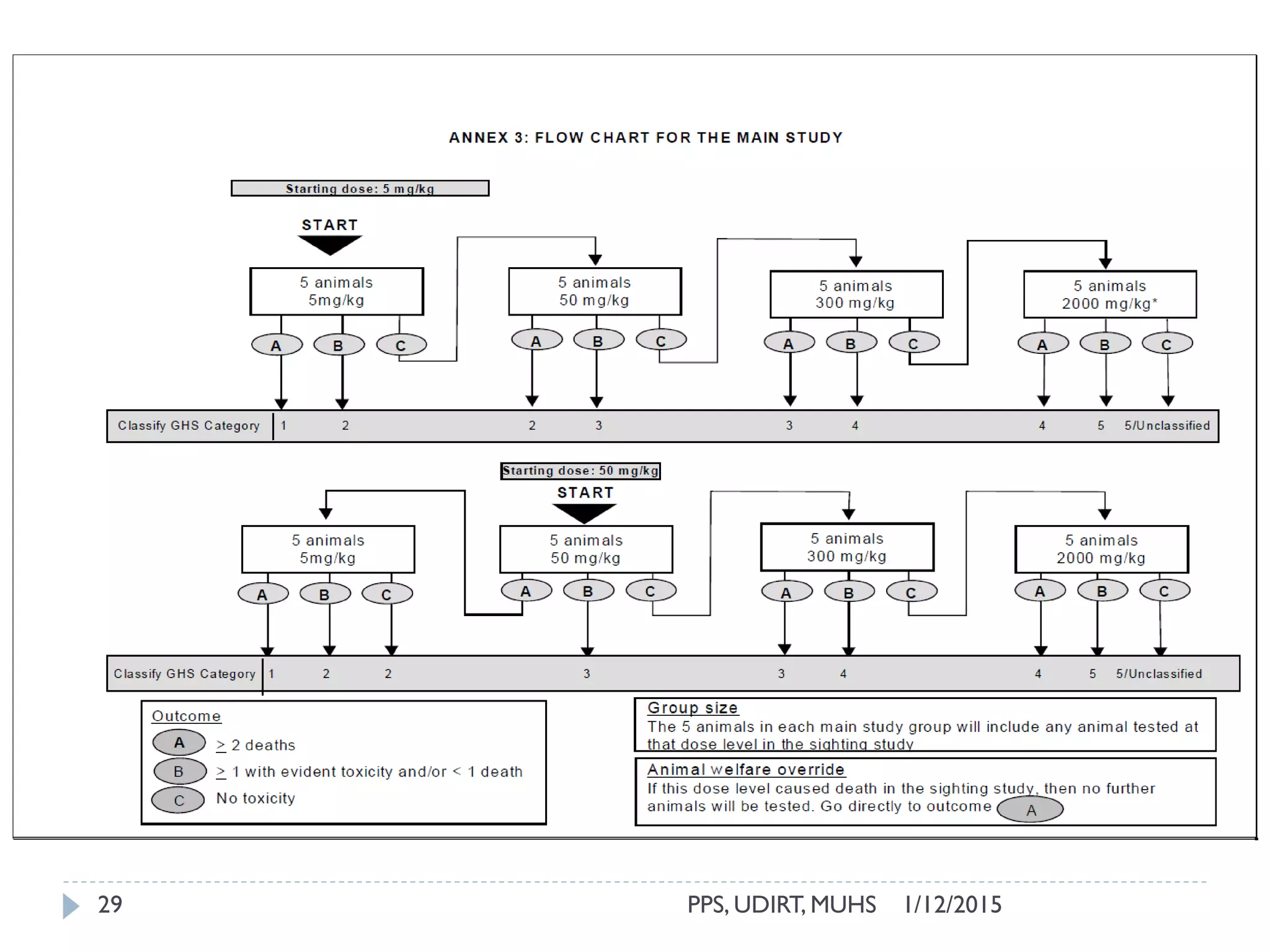
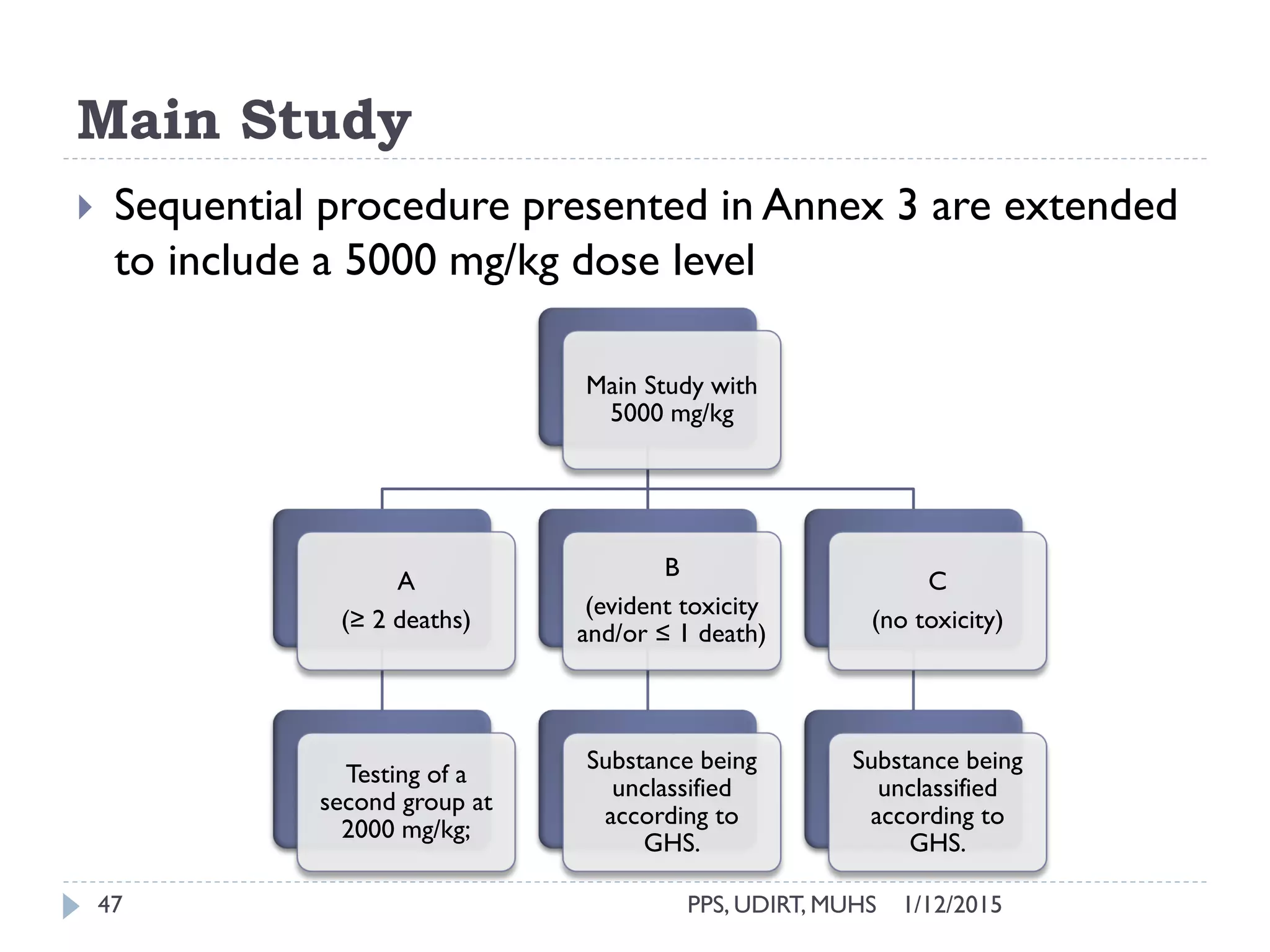
The document outlines the OECD guideline 420 procedure for assessing acute oral toxicity using a fixed dose method, which was adopted to minimize animal suffering by avoiding endpoints based on death. It describes the methodology, including dosing, animal selection, and observation protocols, along with considerations for humane treatment of test subjects. Additionally, it emphasizes the importance of preliminary and limit testing to ensure safety and compliance with global toxicity classification systems.