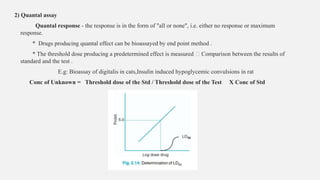
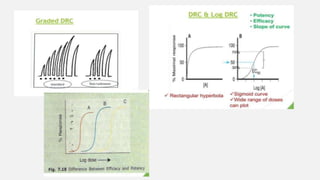
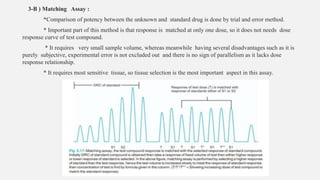
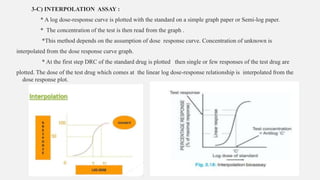
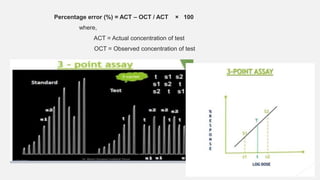
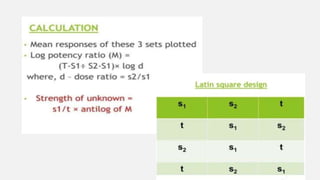
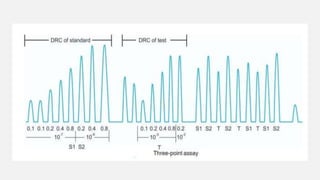
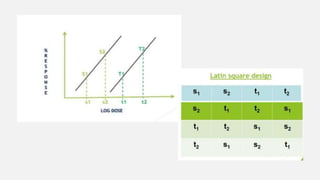
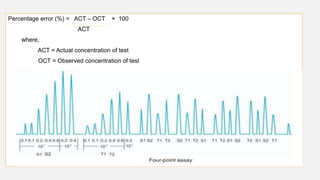
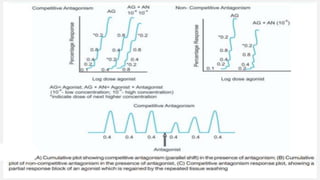
This document provides an overview of bioassay, which is defined as the comparative assessment of the potency of a test compound relative to a standard compound based on their effects on living tissue. It discusses the objectives, principles, types of errors, important criteria, and applications of bioassays. Various types of bioassays are described including direct endpoint assays, quantal assays, and graded assays such as bracketing, matching, interpolation, and multiple point (3-point, 4-point, 6-point) assays. The document explains the procedures, calculations, and importance of different bioassay methods.