Embed presentation
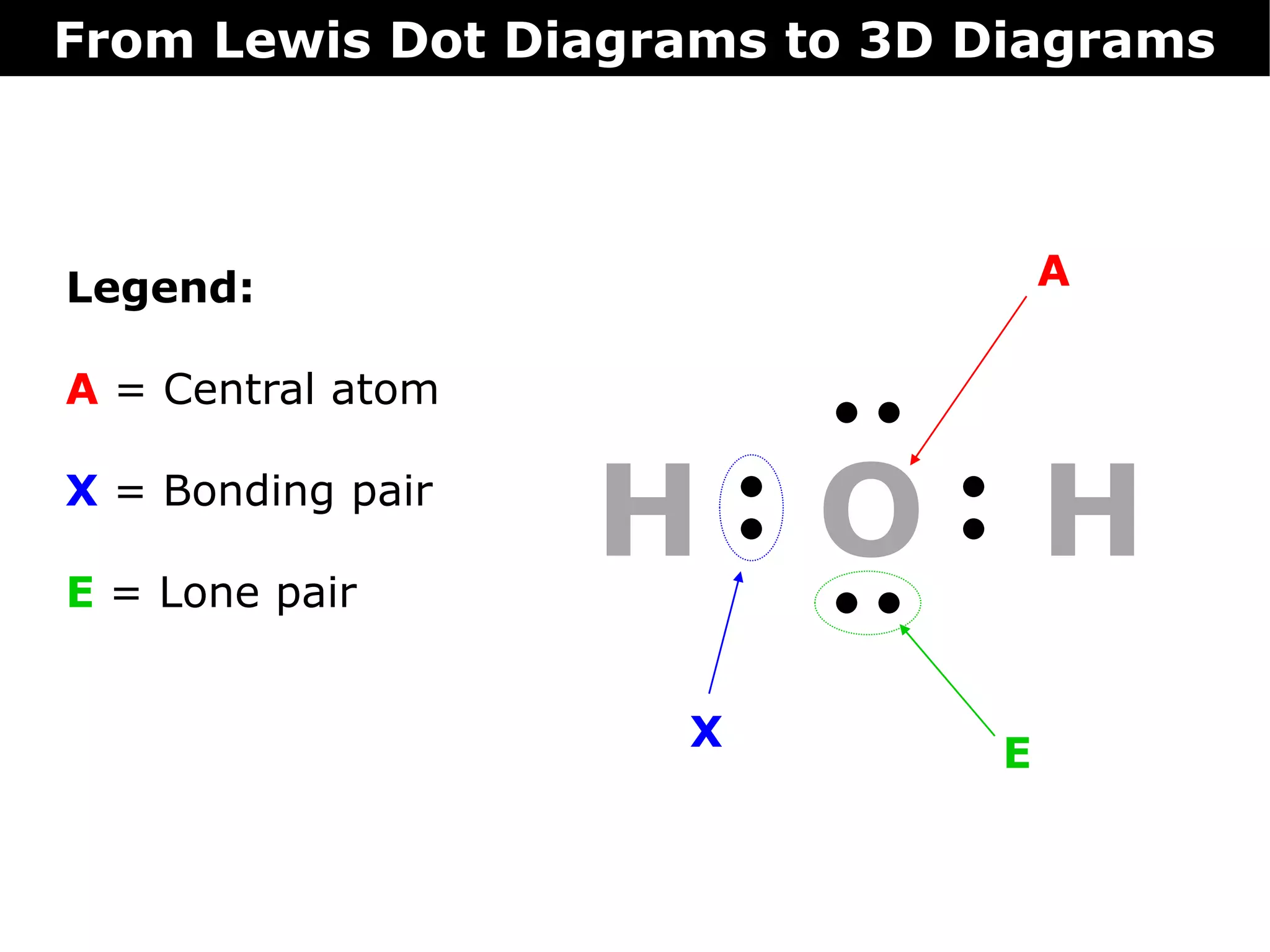
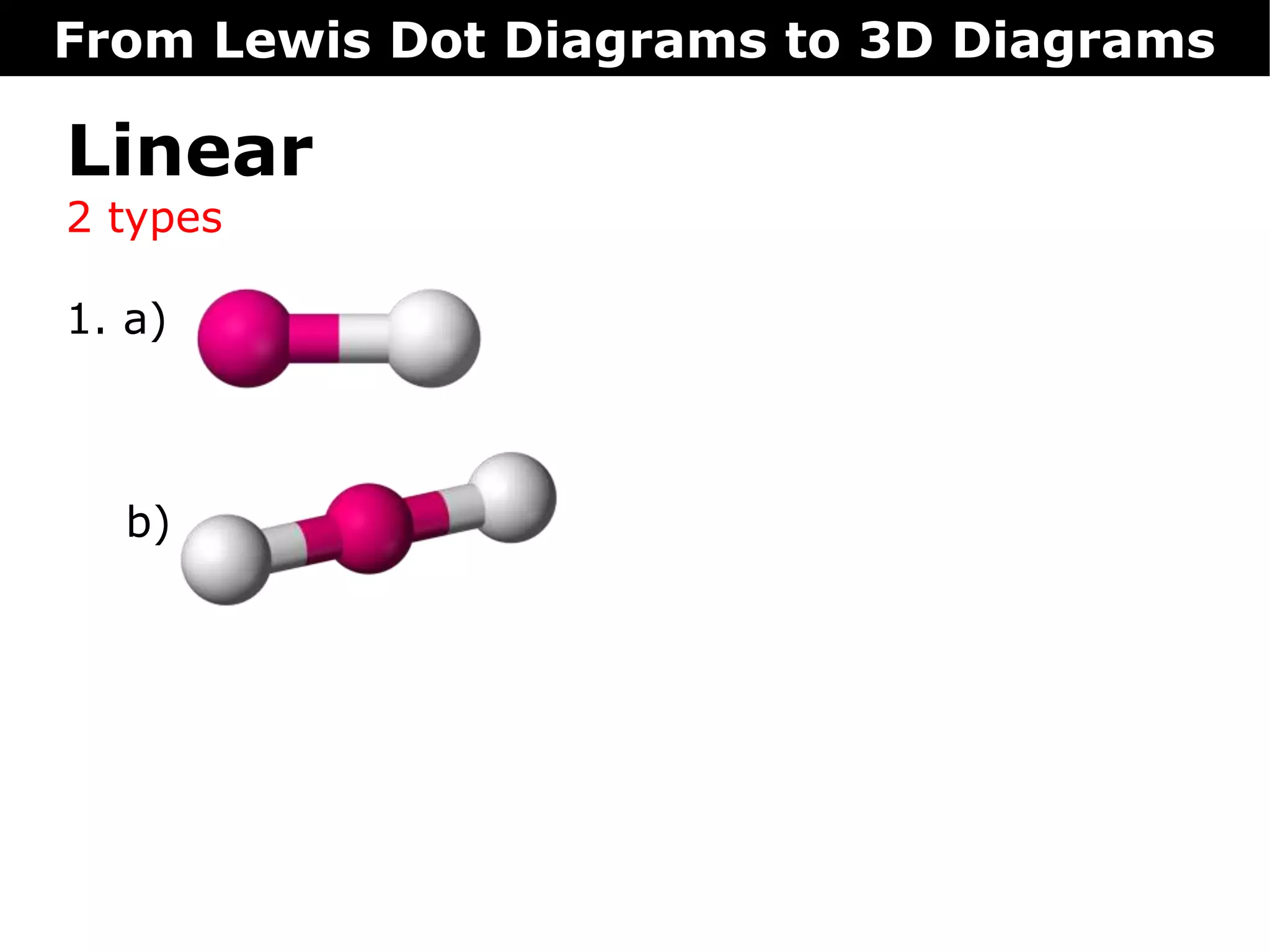
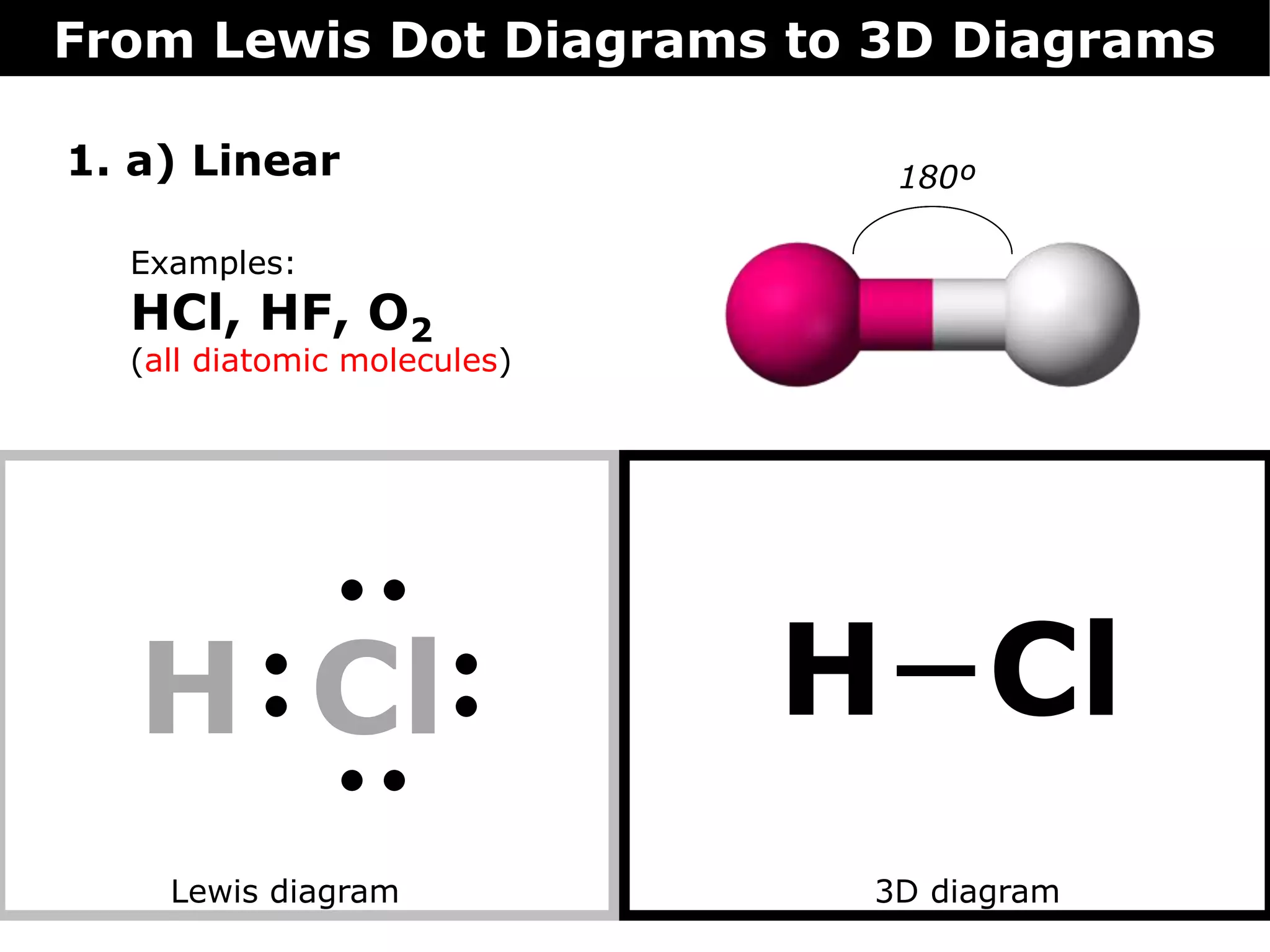
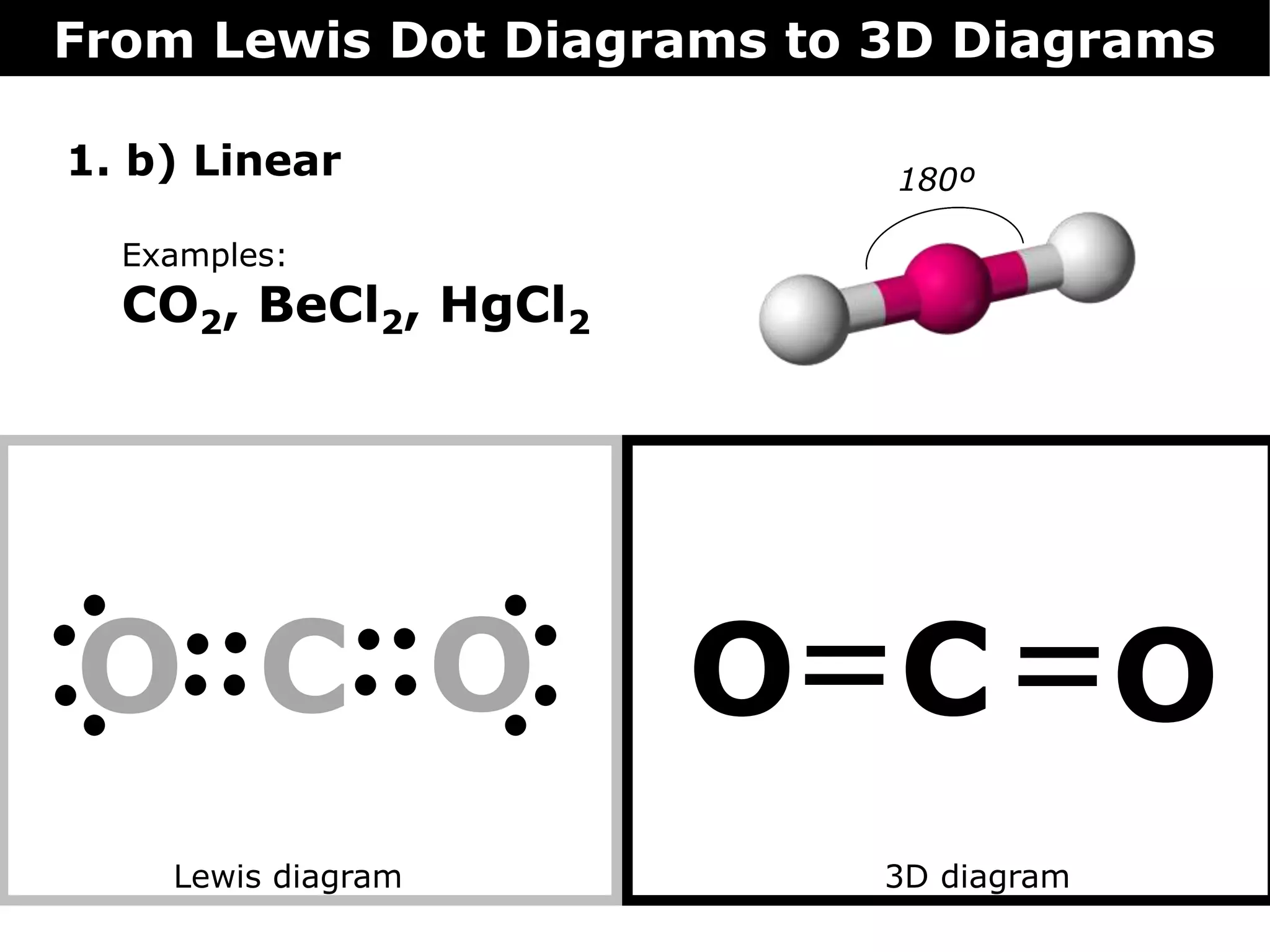
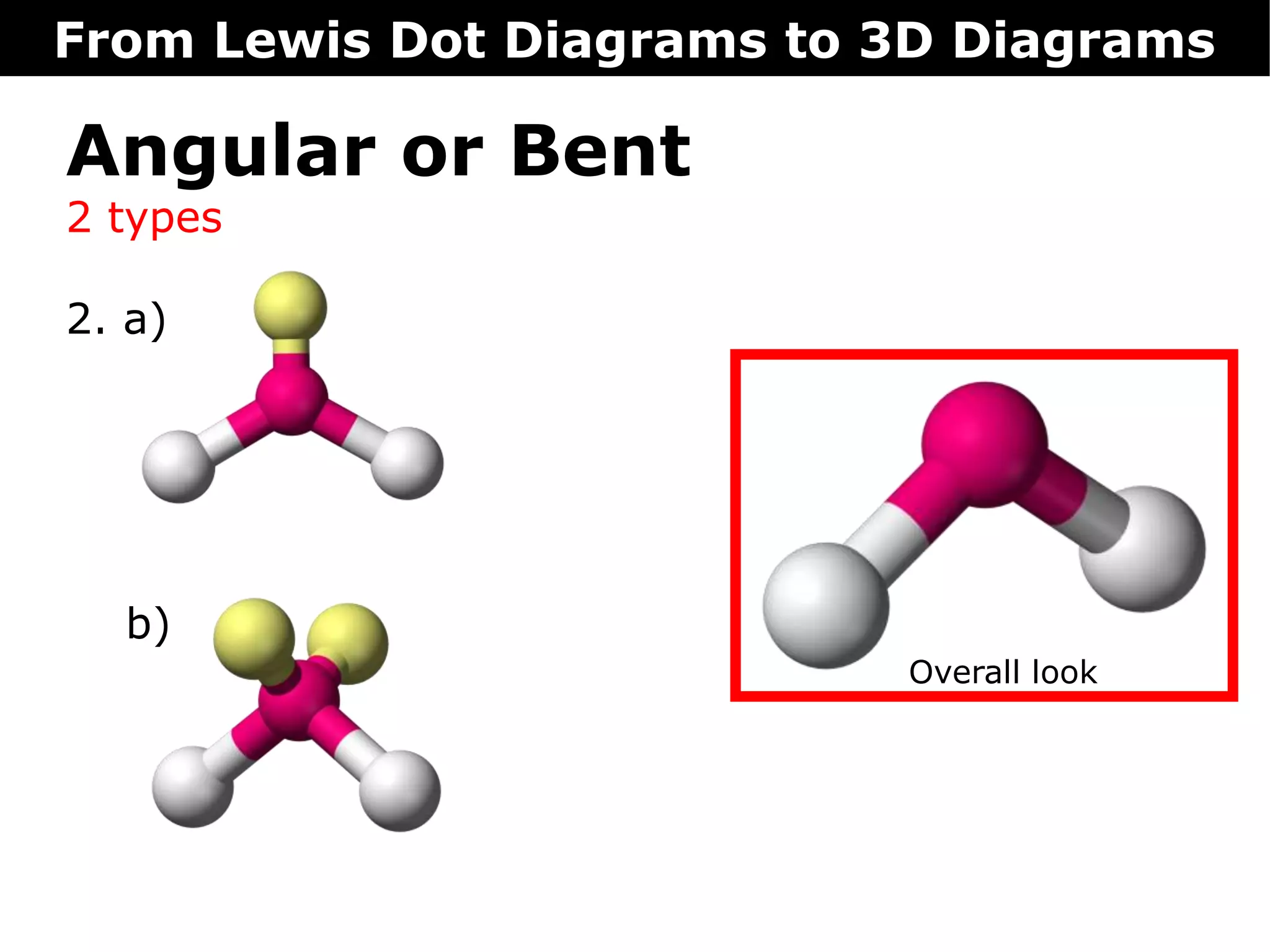
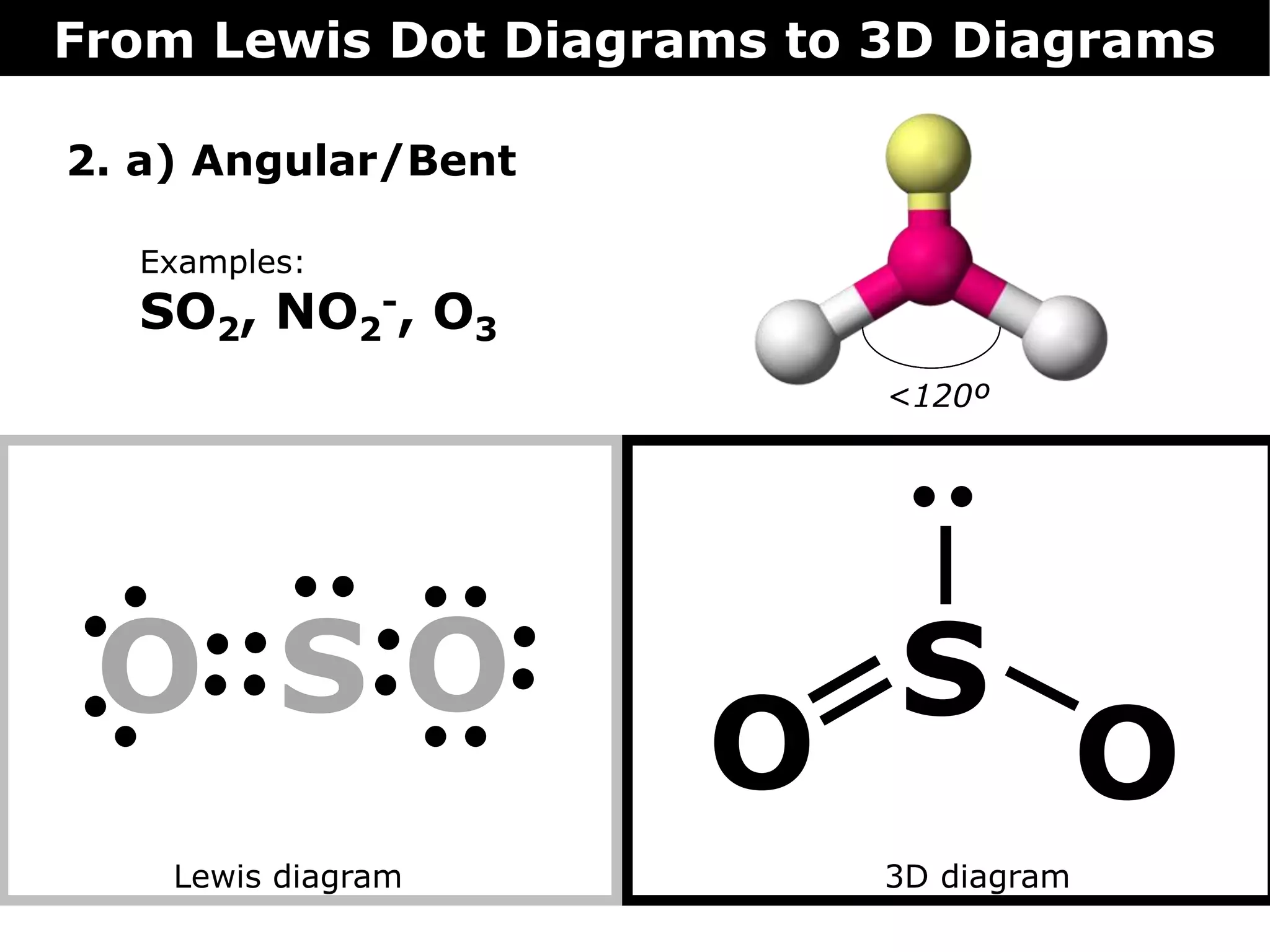
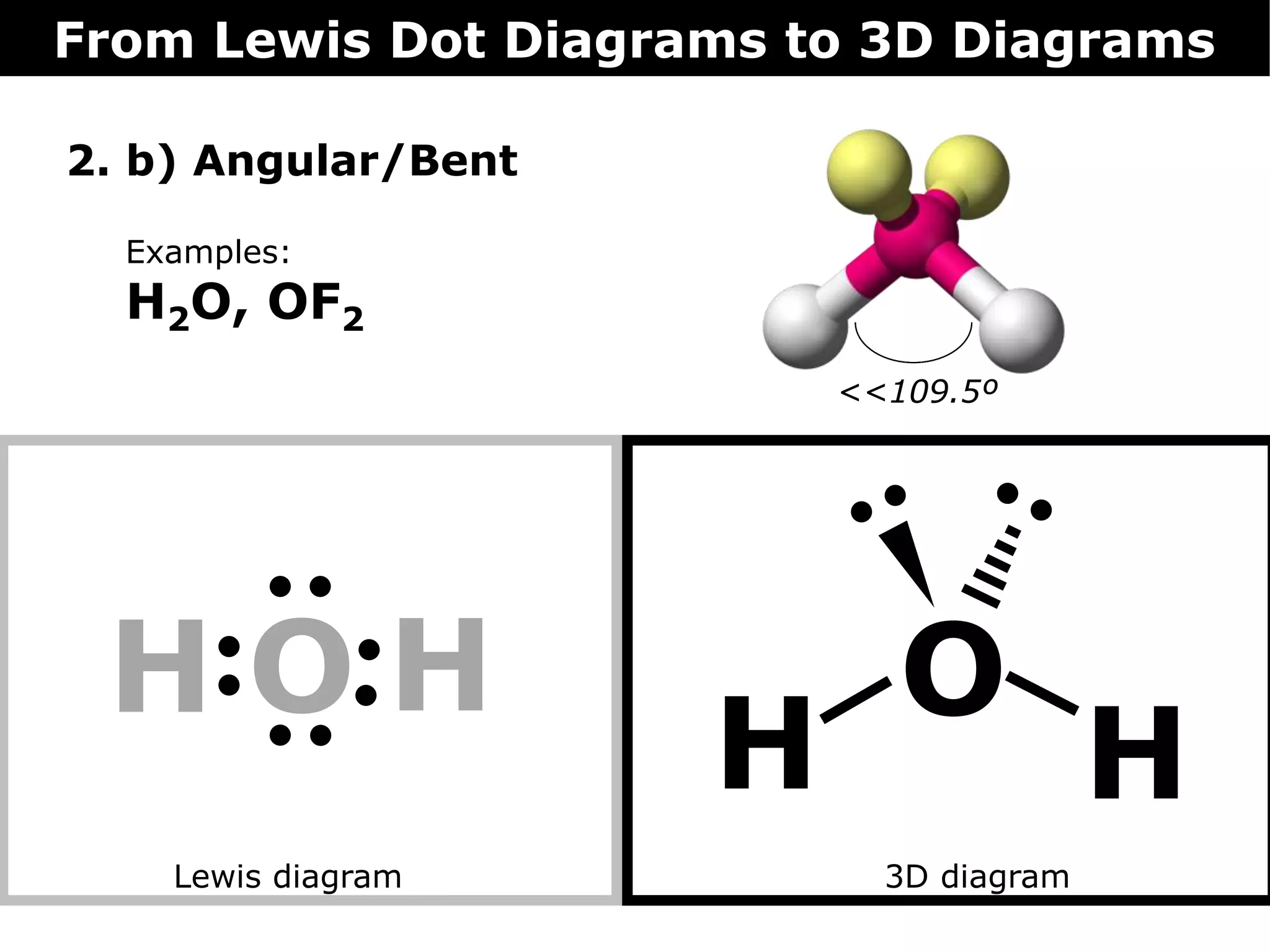
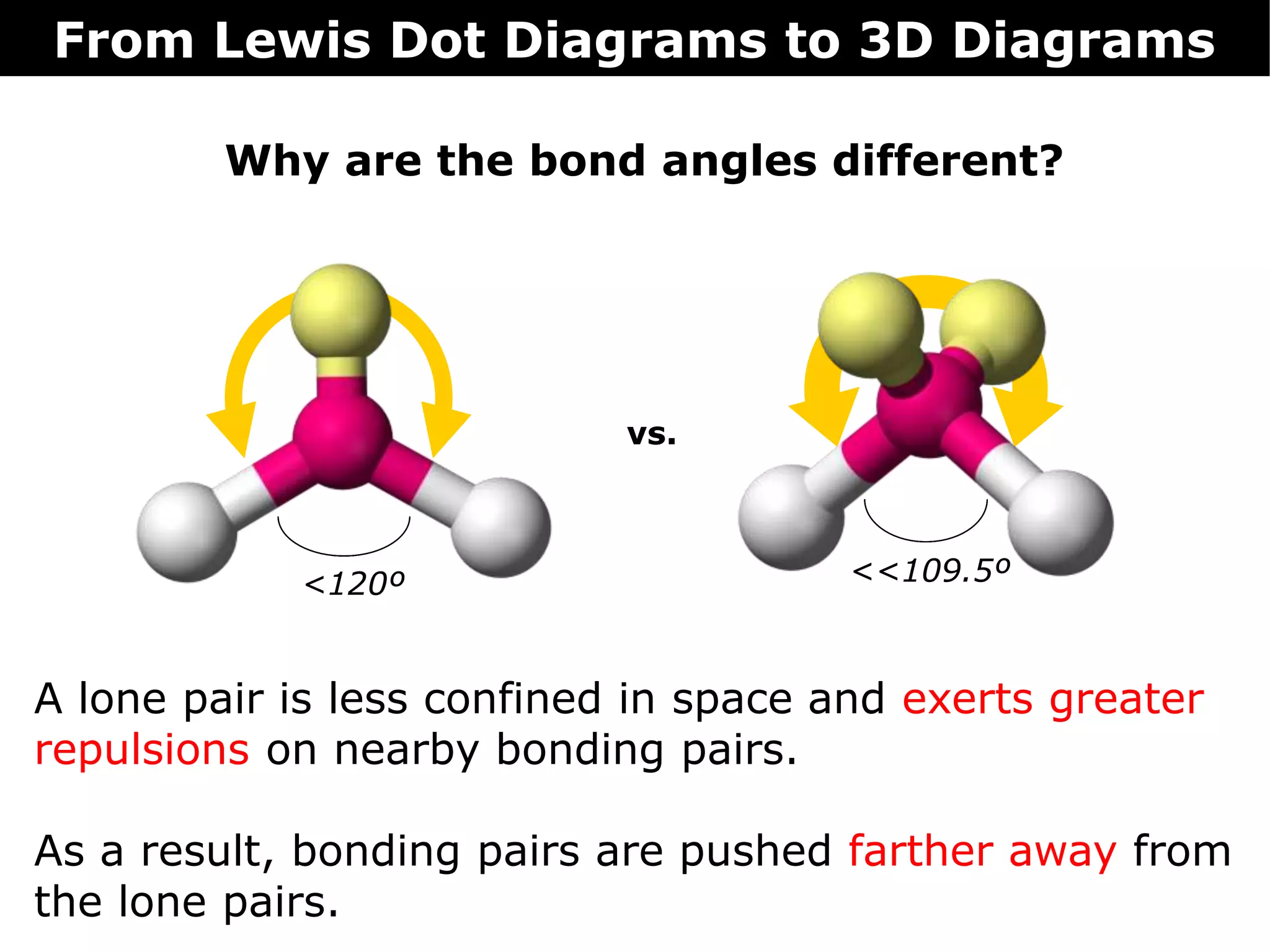
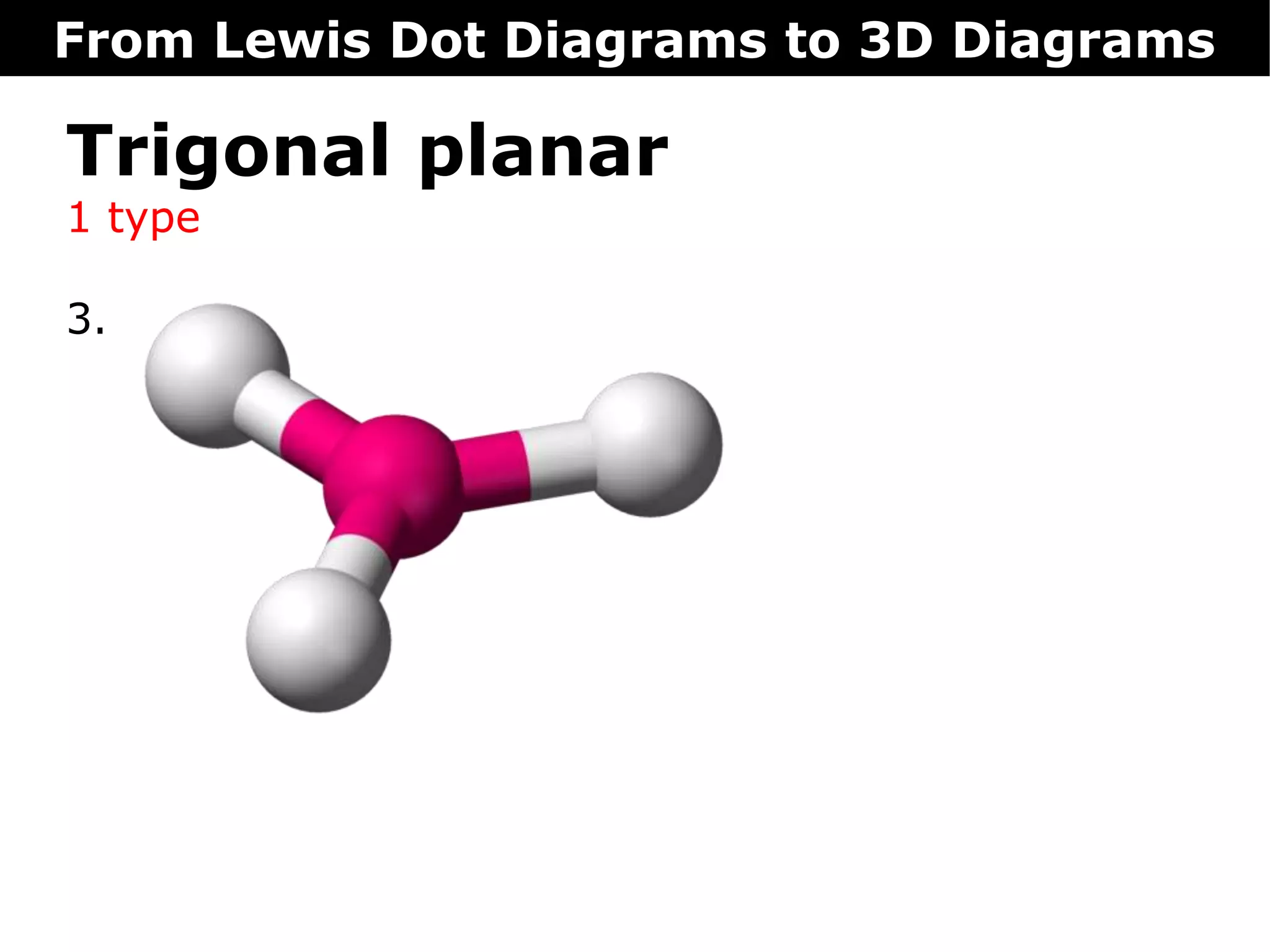
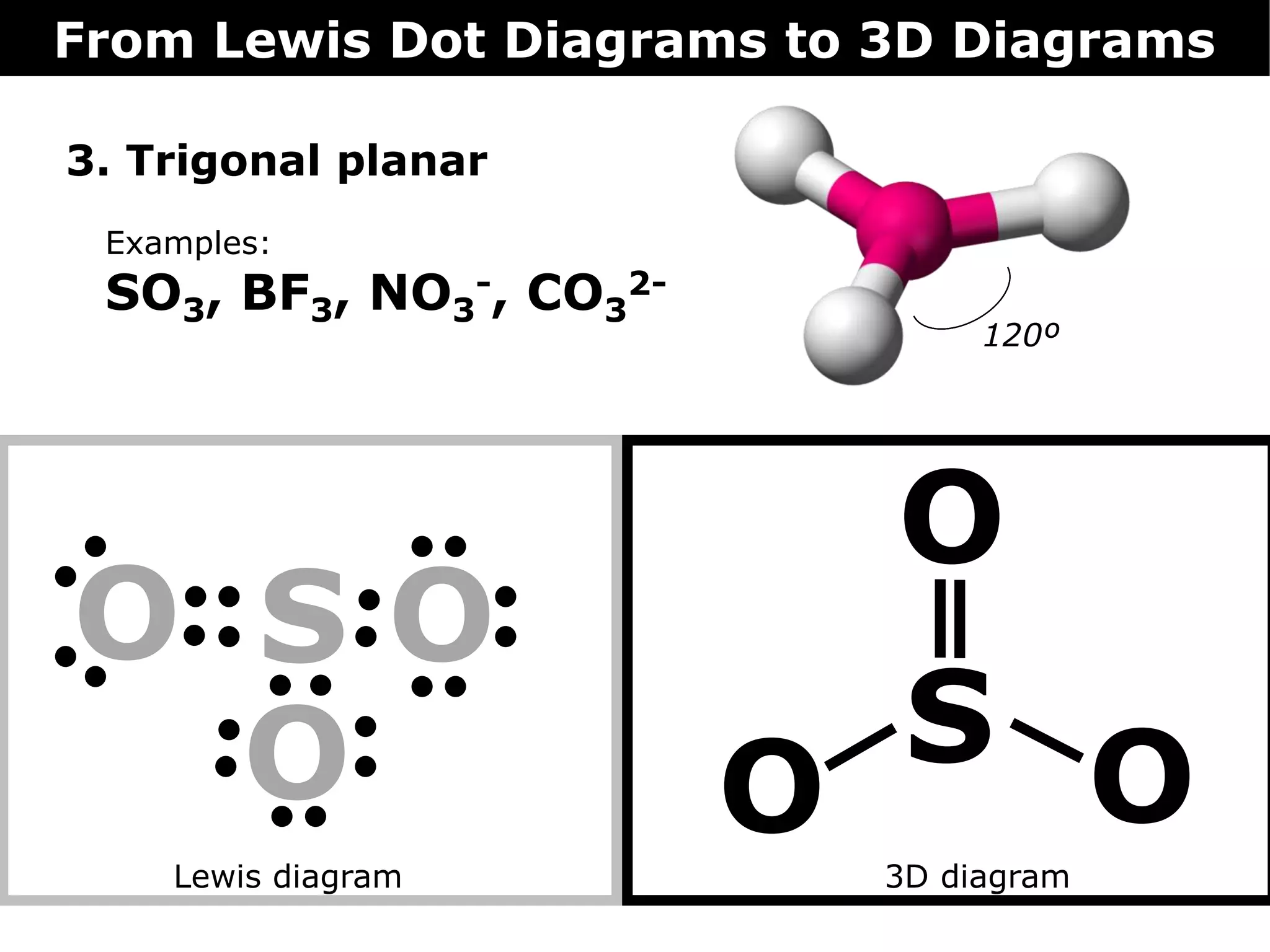
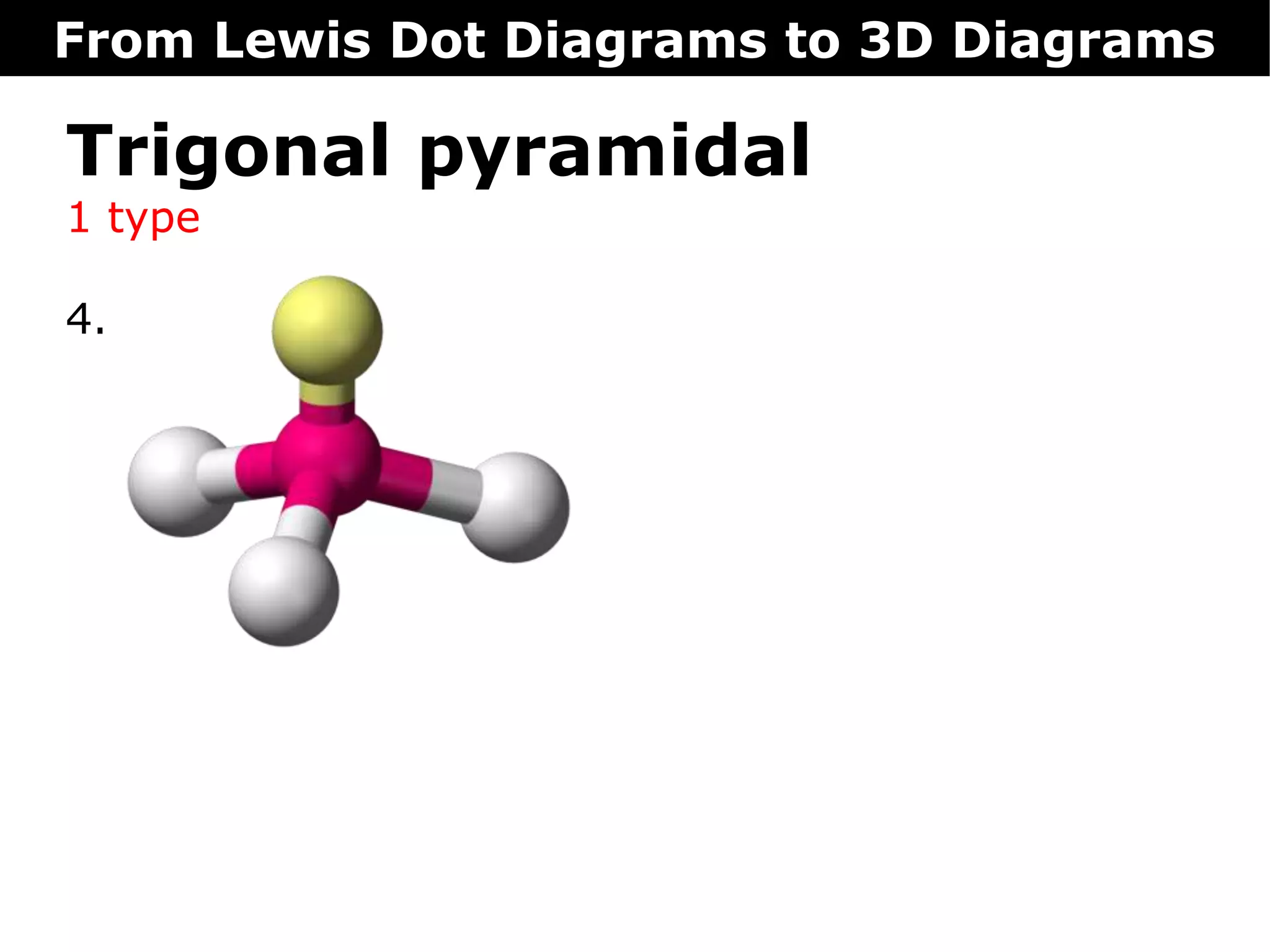
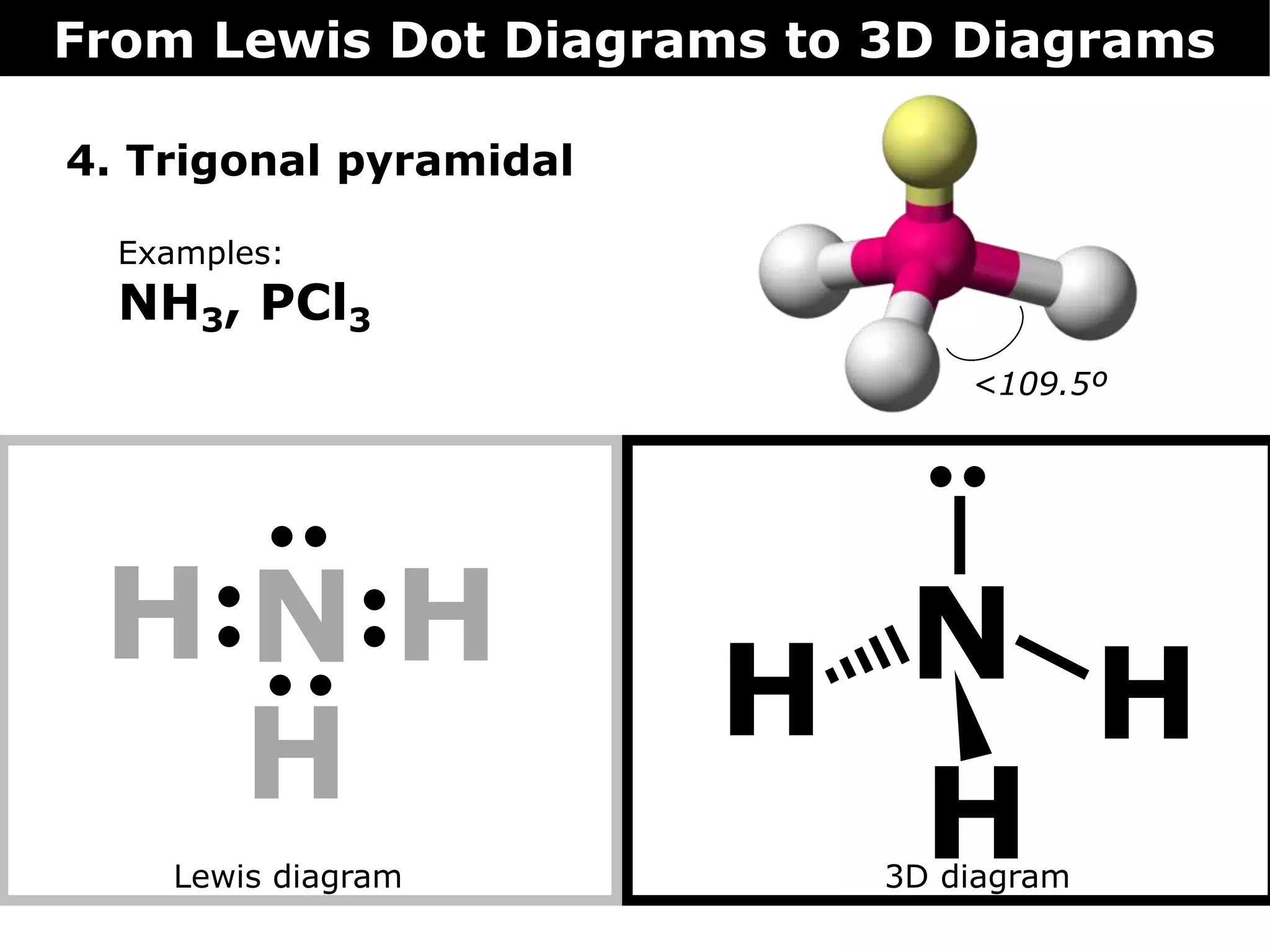
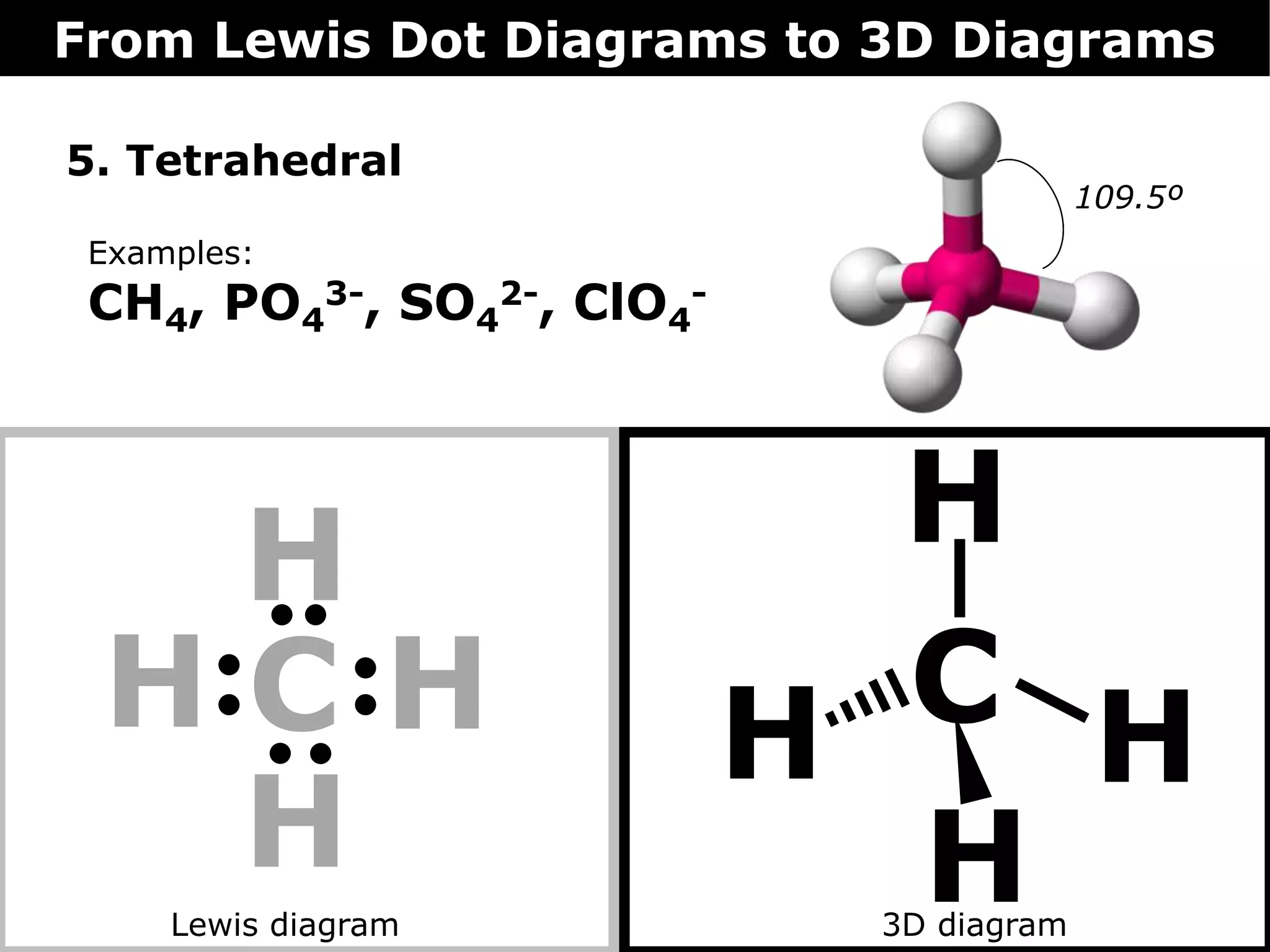
The document discusses how Lewis dot diagrams can be used to predict the 3D structure of molecules. It describes 5 main types of molecular geometries: 1) linear, 2) angular/bent, 3) trigonal planar, 4) trigonal pyramidal, and 5) tetrahedral. Each geometry has characteristic bond angles that are determined by the number of bonding pairs and lone pairs on the central atom. Examples are provided for common molecules that exhibit each type of 3D structure.














