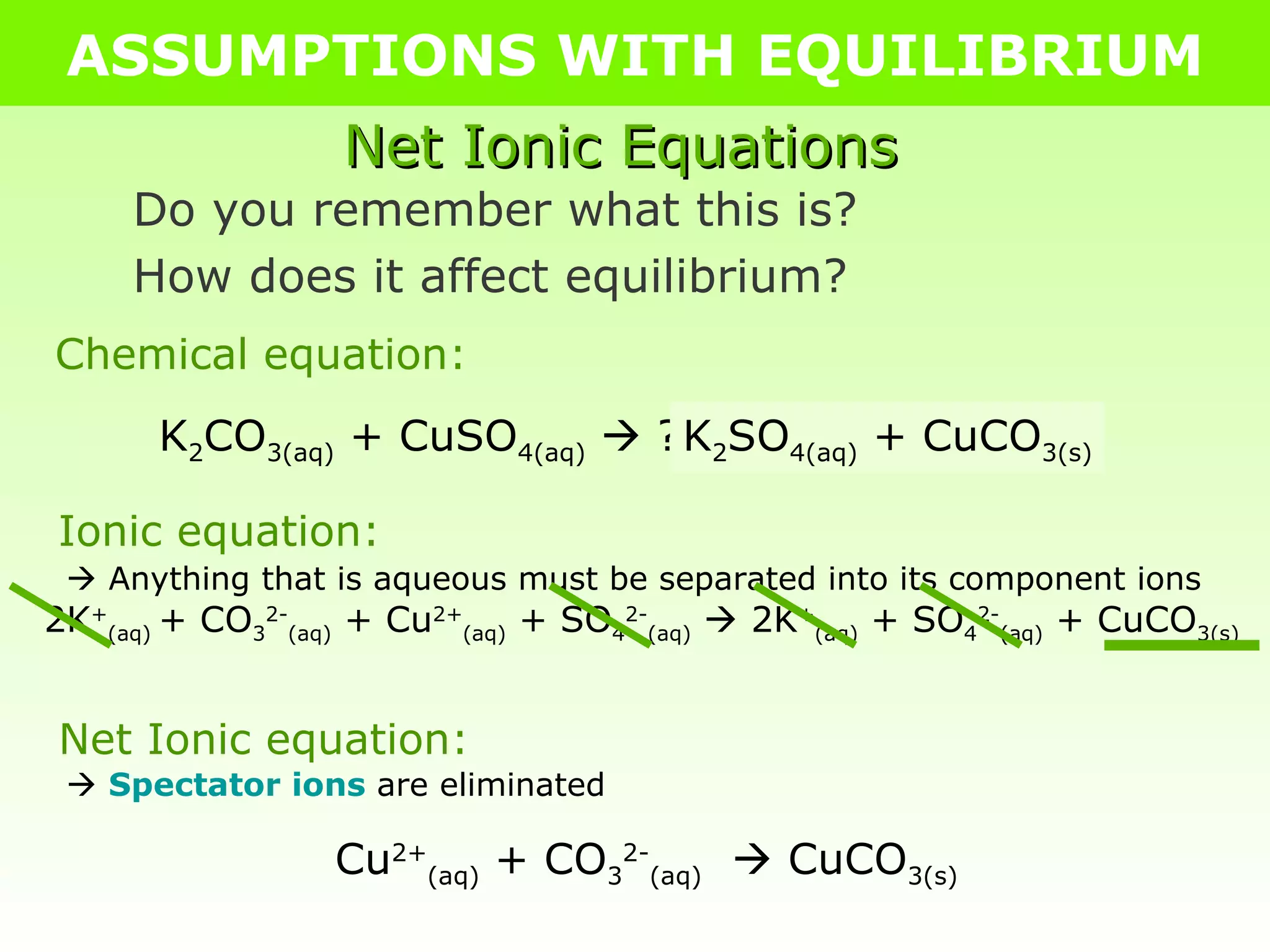
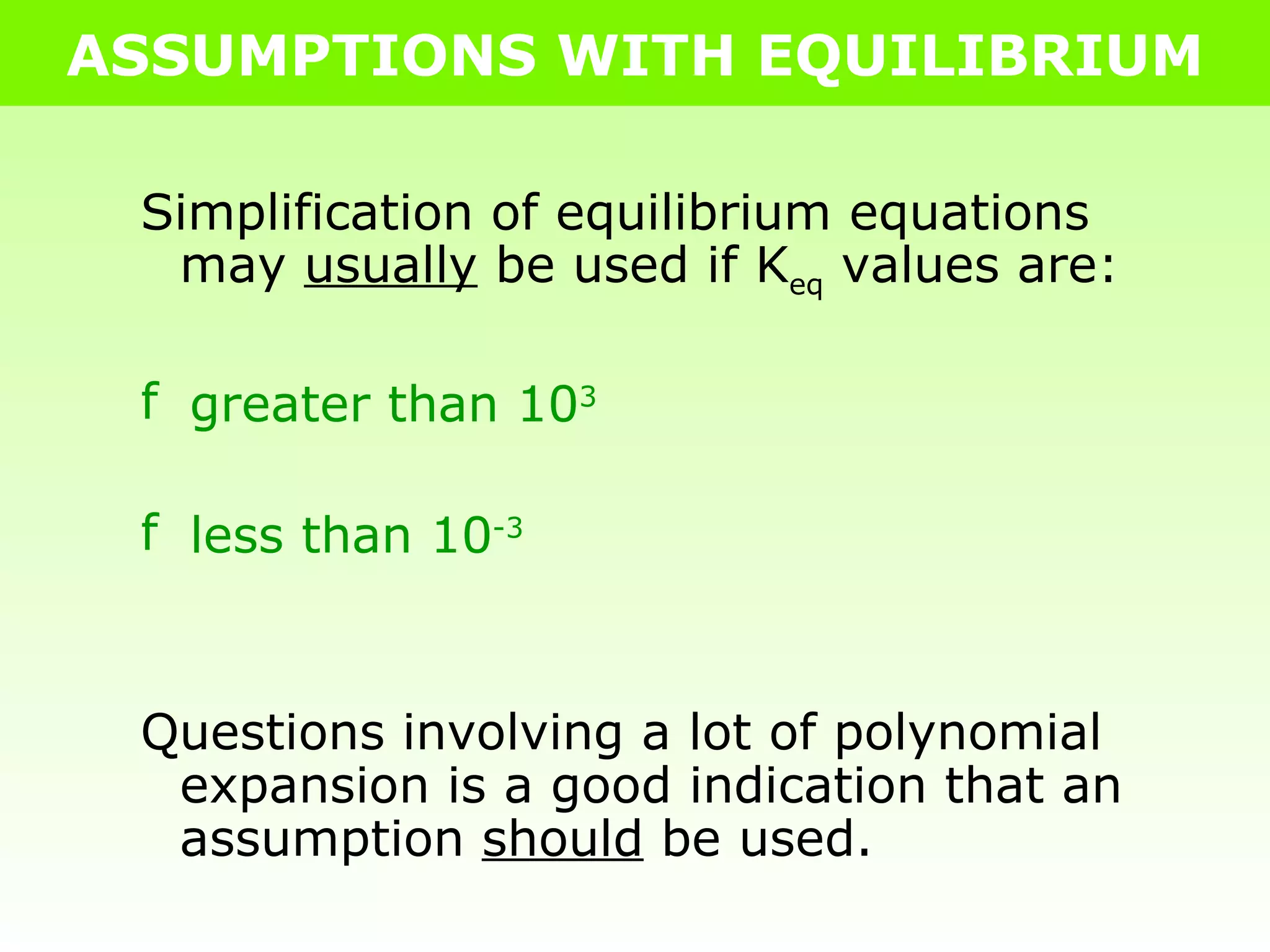
The document discusses making assumptions to simplify equilibrium calculations when the equilibrium constant (Keq) is extremely small or large. It provides an example of calculating equilibrium concentrations for the decomposition of CO2 at 2000°C assuming negligible decomposition due to the small Keq value of 6.40 x 10-7. The assumptions allow obtaining approximate equilibrium concentrations without solving the full cubic equation.

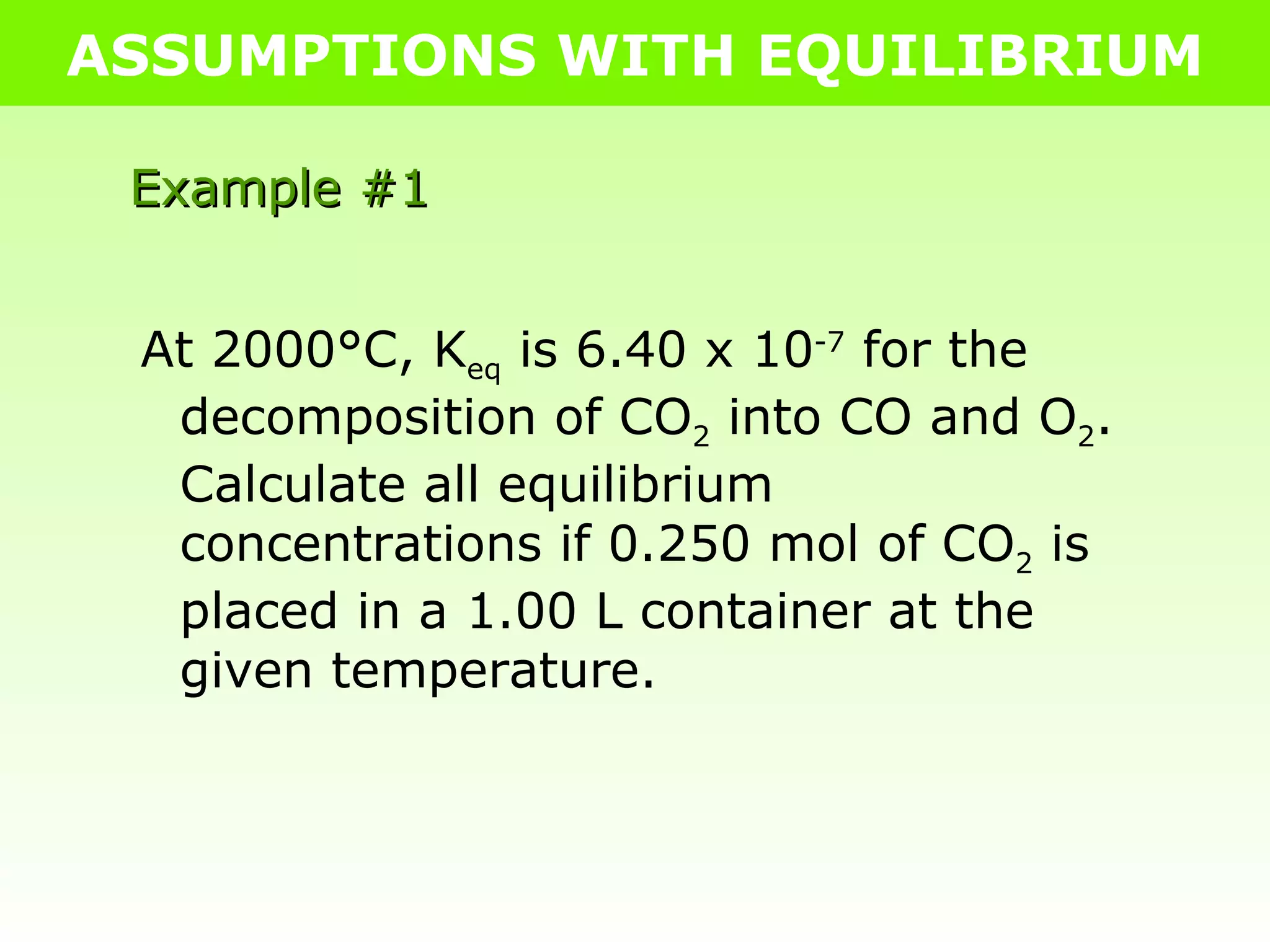
![ASSUMPTIONS WITH EQUILIBRIUM Example #1 At 2000°C, K eq is 6.40 x 10 -7 for the decomposition of CO 2 into CO and O 2 . Calculate all equilibrium concentrations if 0.250 mol of CO 2 is placed in a 1.00 L container at the given temperature. 2CO 2(g) 2CO (g) + O 2(g) I 0.250 0 0 C -2x +2x +x E 0.250-2x 2x x K eq = [CO] 2 [O 2 ] [CO 2 ] 2 6.40x10 -7 = [2x] 2 [x] [0.250-2x] 2 6.40x10 -7 = 4x 3 0.0625-x+4x 2 How do we solve this cubic equation?!](https://image.slidesharecdn.com/tang06-assumptionswithequilibrium2-111017141221-phpapp02/75/Tang-06-assumptions-with-equilibrium-2-3-2048.jpg)

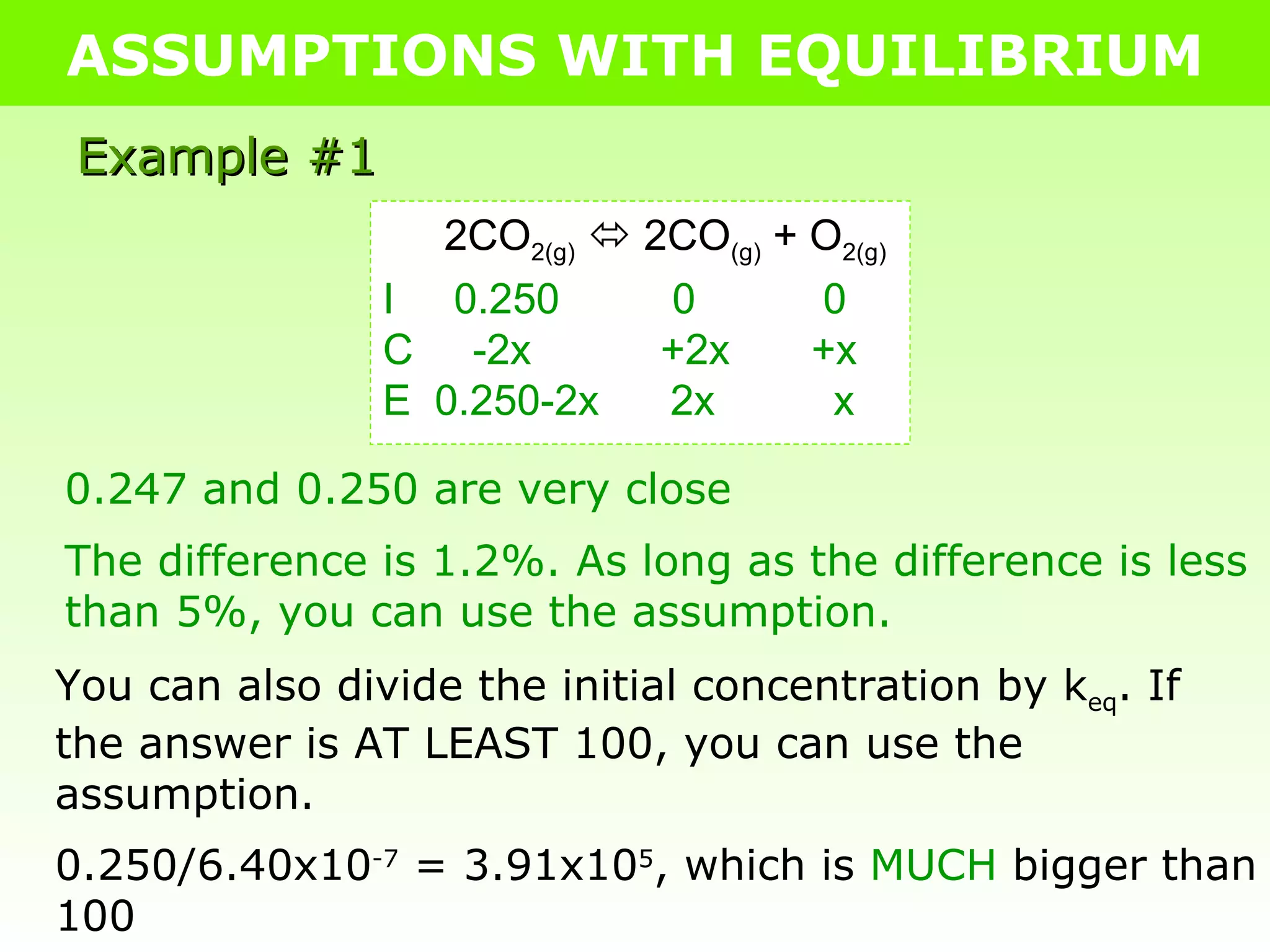
![ASSUMPTIONS WITH EQUILIBRIUM Example #1 2CO 2(g) 2CO (g) + O 2(g) I 0.250 0 0 C -2x +2x +x E 0.250-2x 2x x K eq = [CO] 2 [O 2 ] [CO 2 ] 2 6.40x10 -7 = [2x] 2 [x] [0.250-2x] 2 Assumption: 0.250-2x = 0.250 6.40x10 -7 = [2x] 2 [x] [ 0.250 ] 2 6.40x10 -7 = 4x 3 0.0625 4.00x10 -8 = 4x 3 1.00x10 -8 = x 3 3 √(1.00x10 -8 ) = 3 √(x 3 ) 2.15x10 -3 = x](https://image.slidesharecdn.com/tang06-assumptionswithequilibrium2-111017141221-phpapp02/75/Tang-06-assumptions-with-equilibrium-2-5-2048.jpg)
![ASSUMPTIONS WITH EQUILIBRIUM Example #1 At 2000°C, K eq is 6.40 x 10 -7 for the decomposition of CO 2 into CO and O 2 . Calculate all equilibrium concentrations if 0.250 mol of CO 2 is placed in a 1.00 L container at the given temperature. 2CO 2(g) 2CO (g) + O 2(g) I 0.250 0 0 C -2x +2x +x E 0.250-2x 2x x x = 2.15x10 -3 =2(2.15x10 -3 ) =0.0043M =0.250 – 2(2.15x10 -3 ) =0.247M .: [CO 2(g) ] = 2.47x10 -1 M, [CO (g) ] = 4.30x10 -3 M, & [O 2(g) ] = 4.30x10 -3 M](https://image.slidesharecdn.com/tang06-assumptionswithequilibrium2-111017141221-phpapp02/75/Tang-06-assumptions-with-equilibrium-2-6-2048.jpg)
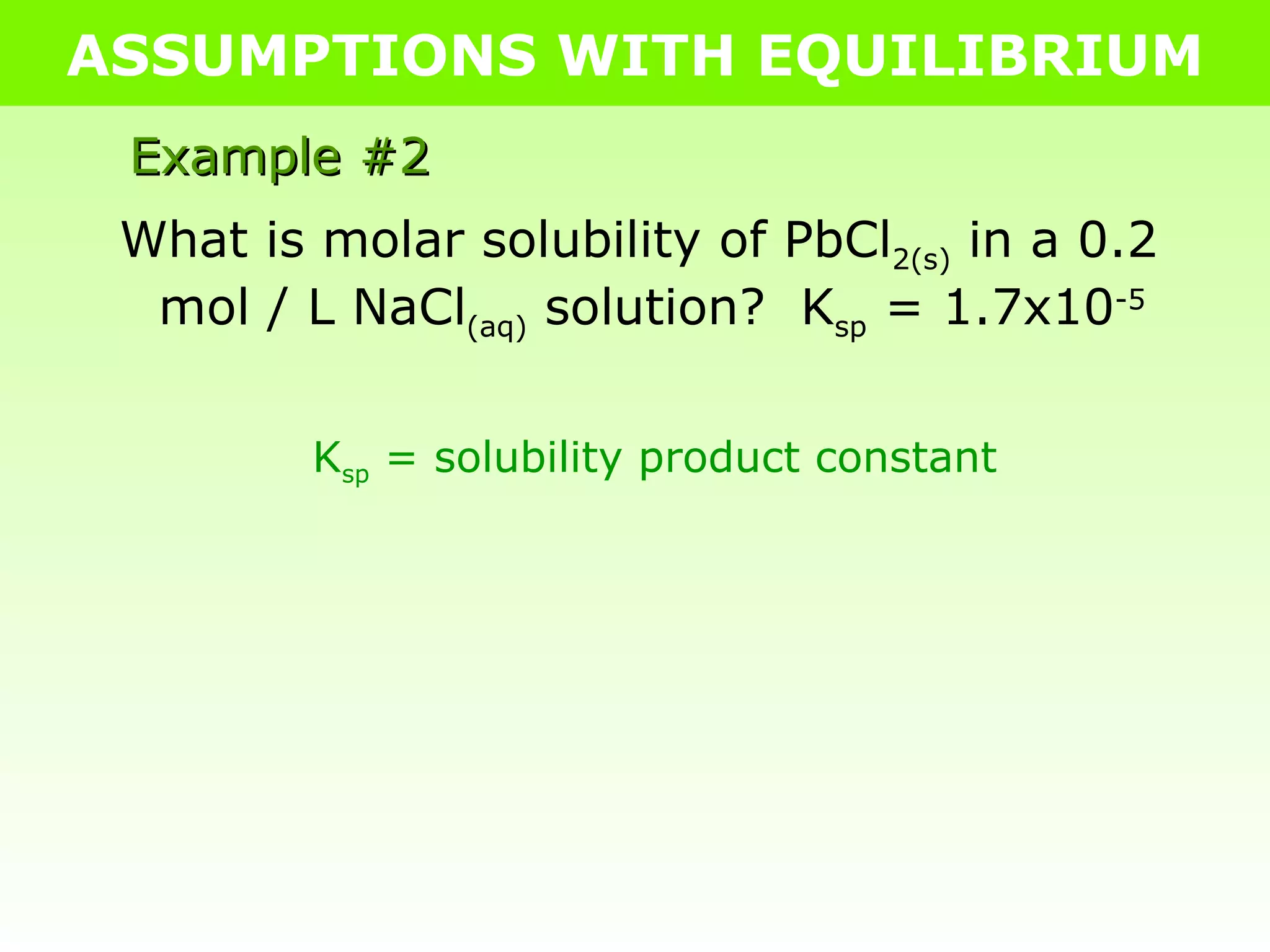
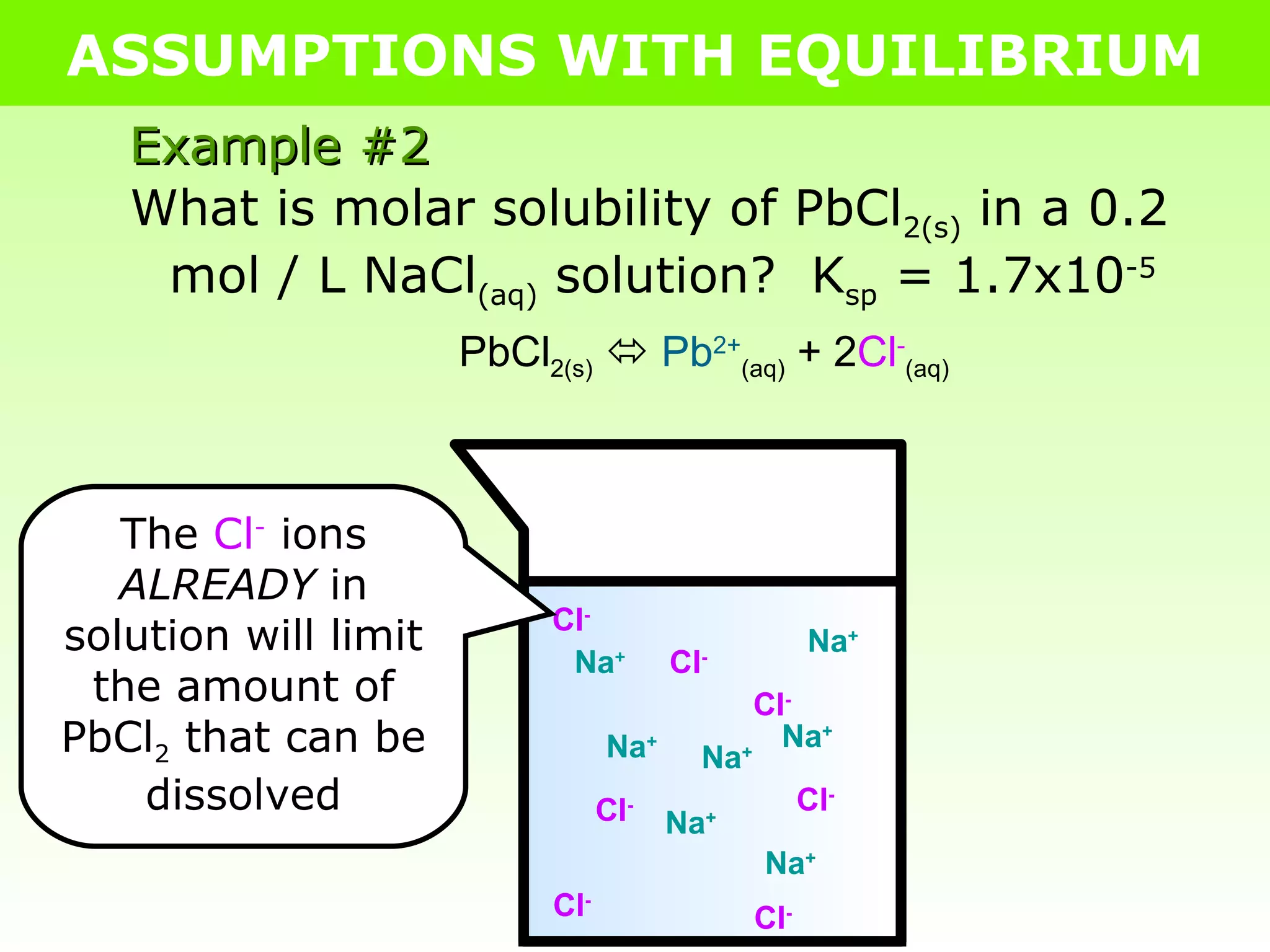
![ASSUMPTIONS WITH EQUILIBRIUM Example #2 What is molar solubility of PbCl 2(s) in a 0.2 mol / L NaCl (aq) solution? K sp = 1.7x10 -5 PbCl 2(s) Pb 2+ (aq) + 2Cl - (aq) I - 0 0.2 C - +x +2x E - x 0.2+2x K sp = [Pb 2+ ][Cl - ] 2 1.7x10 -5 = [x][0.2+2x] 2 Because K sp is SO small, very little PbCl 2 actually dissolves Assumption: 0.2+2x = 0.2 1.7x10 -5 = [x][ 0.2 ] 2 1.7x10 -5 = x 0.04 4.2x10 -4 = x .: the molar solubility of PbCl 2 in 0.2M NaCl (aq) is 4.2x10 -4 mol/L](https://image.slidesharecdn.com/tang06-assumptionswithequilibrium2-111017141221-phpapp02/75/Tang-06-assumptions-with-equilibrium-2-11-2048.jpg)