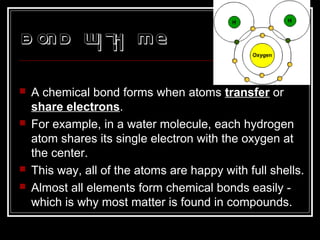
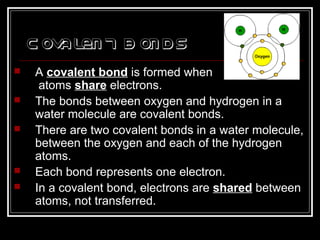
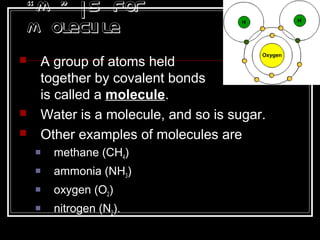
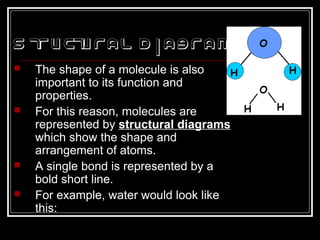
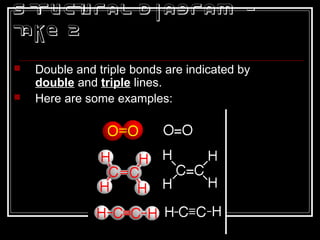
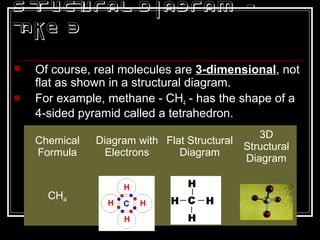
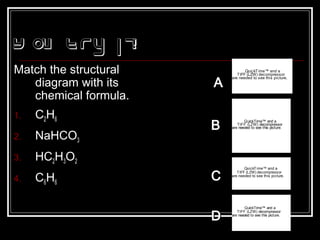
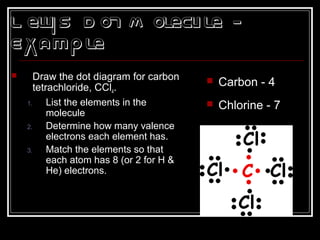
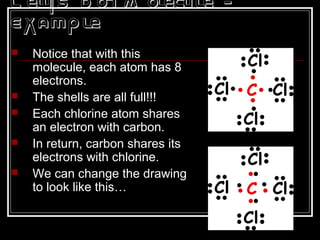
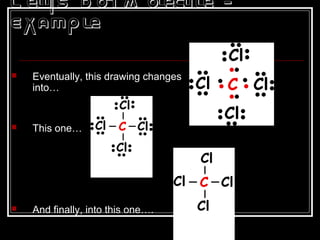
This document discusses covalent bonding and molecular structures. It defines covalent bonds as bonds formed by shared electron pairs between atoms. It explains that molecules are groups of atoms held together by covalent bonds in a specific ratio and shape. The document discusses drawing Lewis dot structures and molecular diagrams to represent molecules and the bonding between their atoms. It provides examples of drawing the Lewis dot structure for carbon tetrachloride and matching molecular diagrams to chemical formulas.