1. Chemical bonds are invisible forces that hold atoms together in compounds. They form when atoms undergo changes and combine with each other.
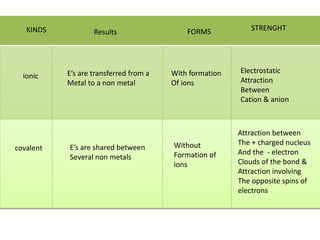
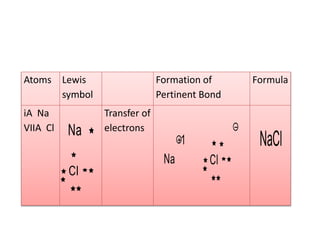
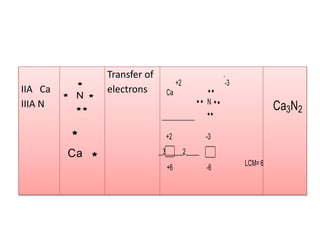
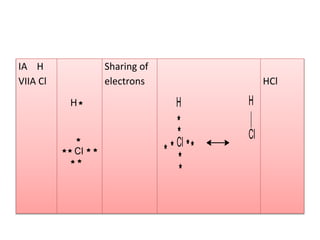
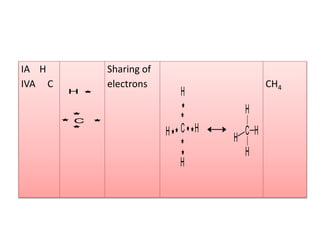
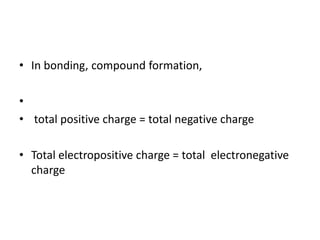
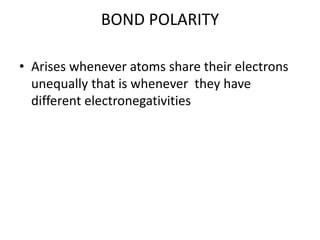
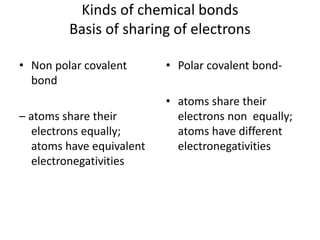
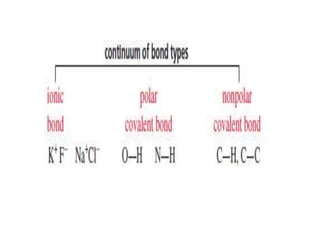
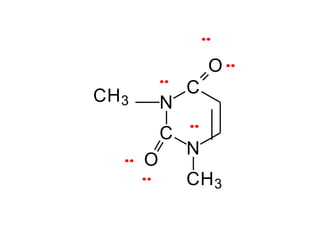
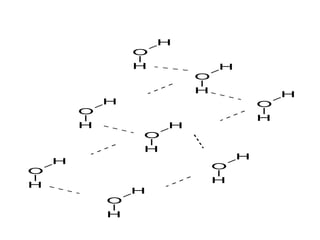
2. There are two main types of chemical bonds - ionic bonds and covalent bonds. Ionic bonds involve the transfer of electrons between atoms to form ions, while covalent bonds involve the sharing of electrons between atoms.
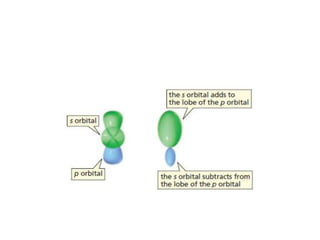
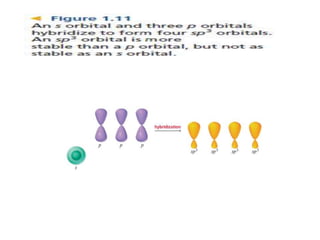
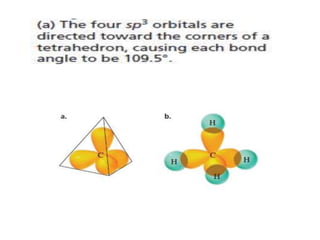
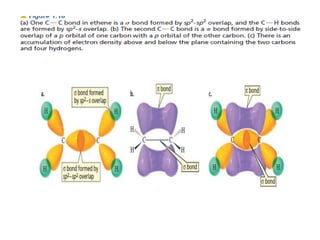
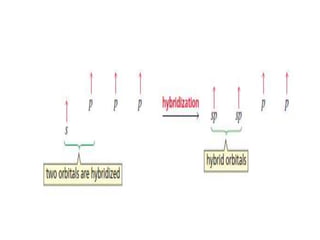
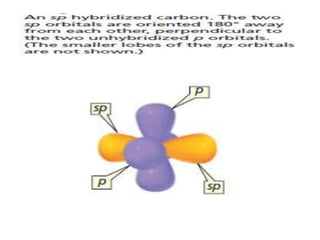
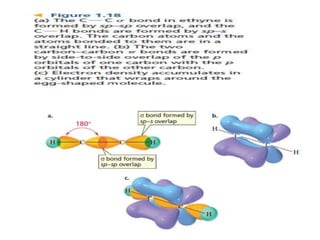
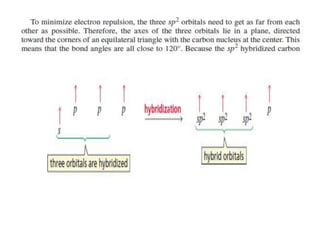
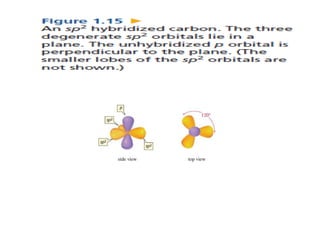
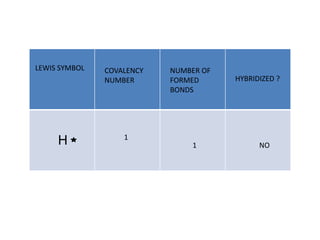
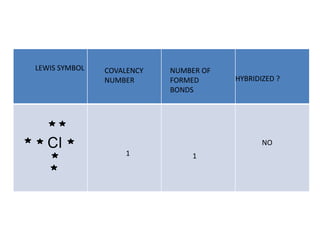
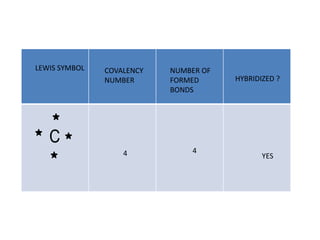
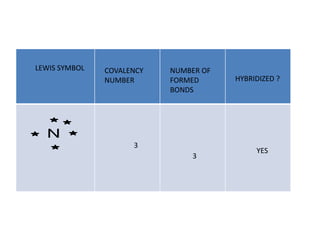
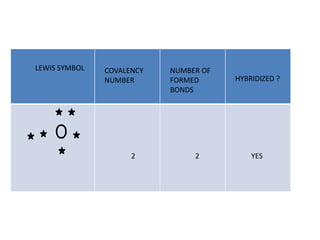
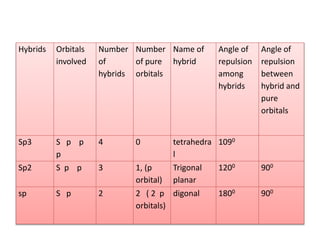
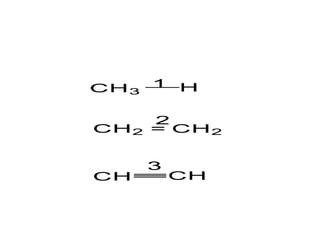
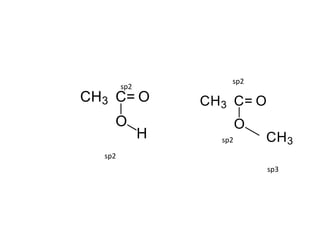
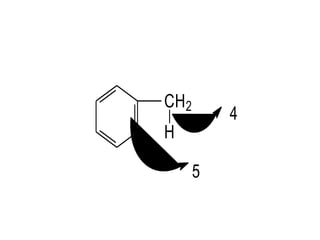
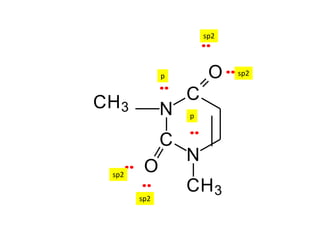
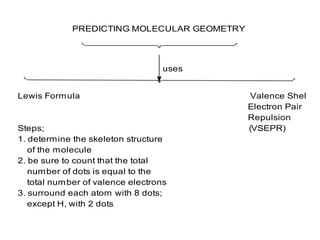
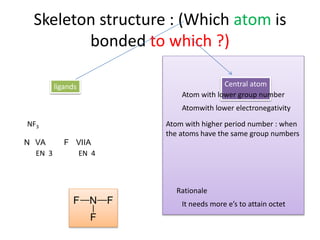
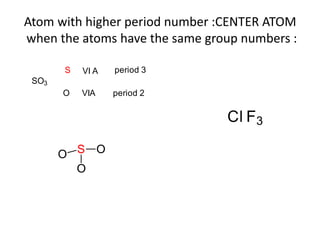
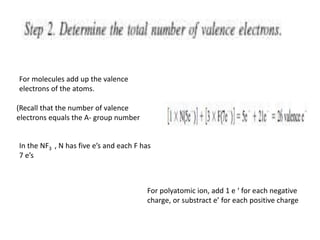
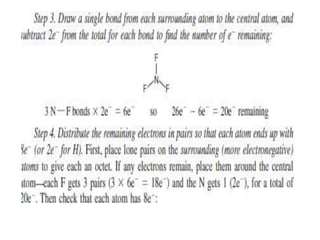
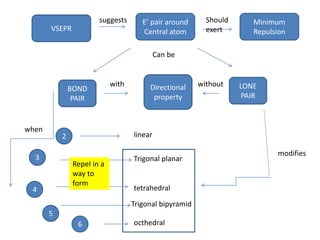
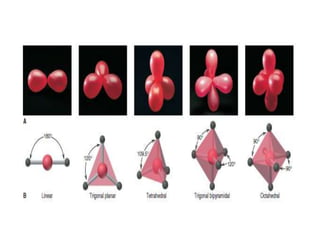
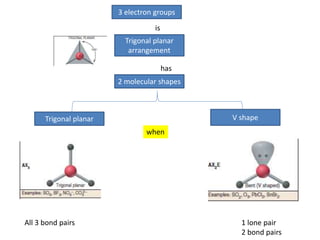
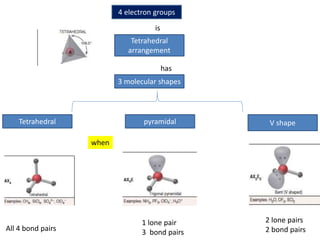
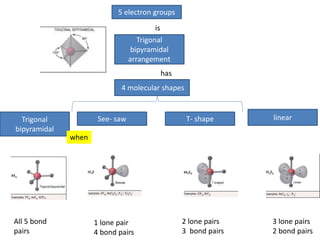
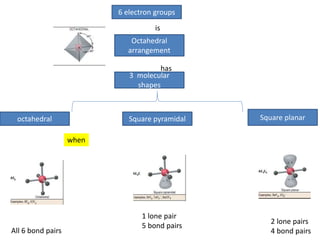
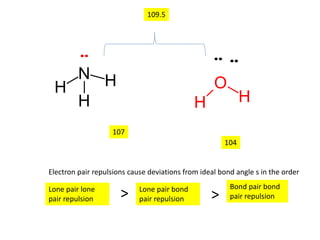
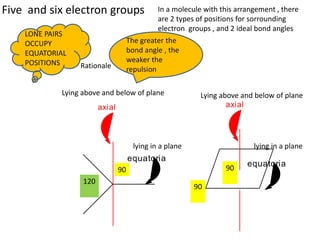
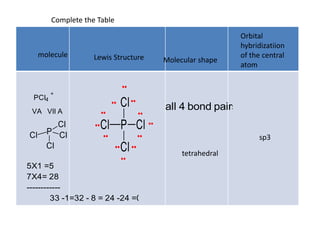
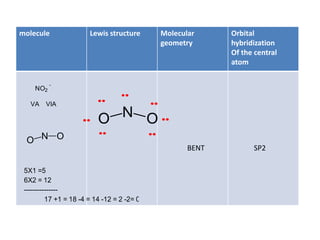
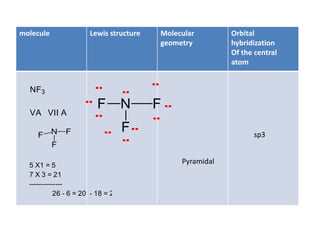
3. The shape and properties of molecules can be predicted using Lewis structures, the valence shell electron pair repulsion (VSEPR) model, and by determining the hybridization of the atoms' orbitals. This allows for determining important characteristics like molecular geometry.