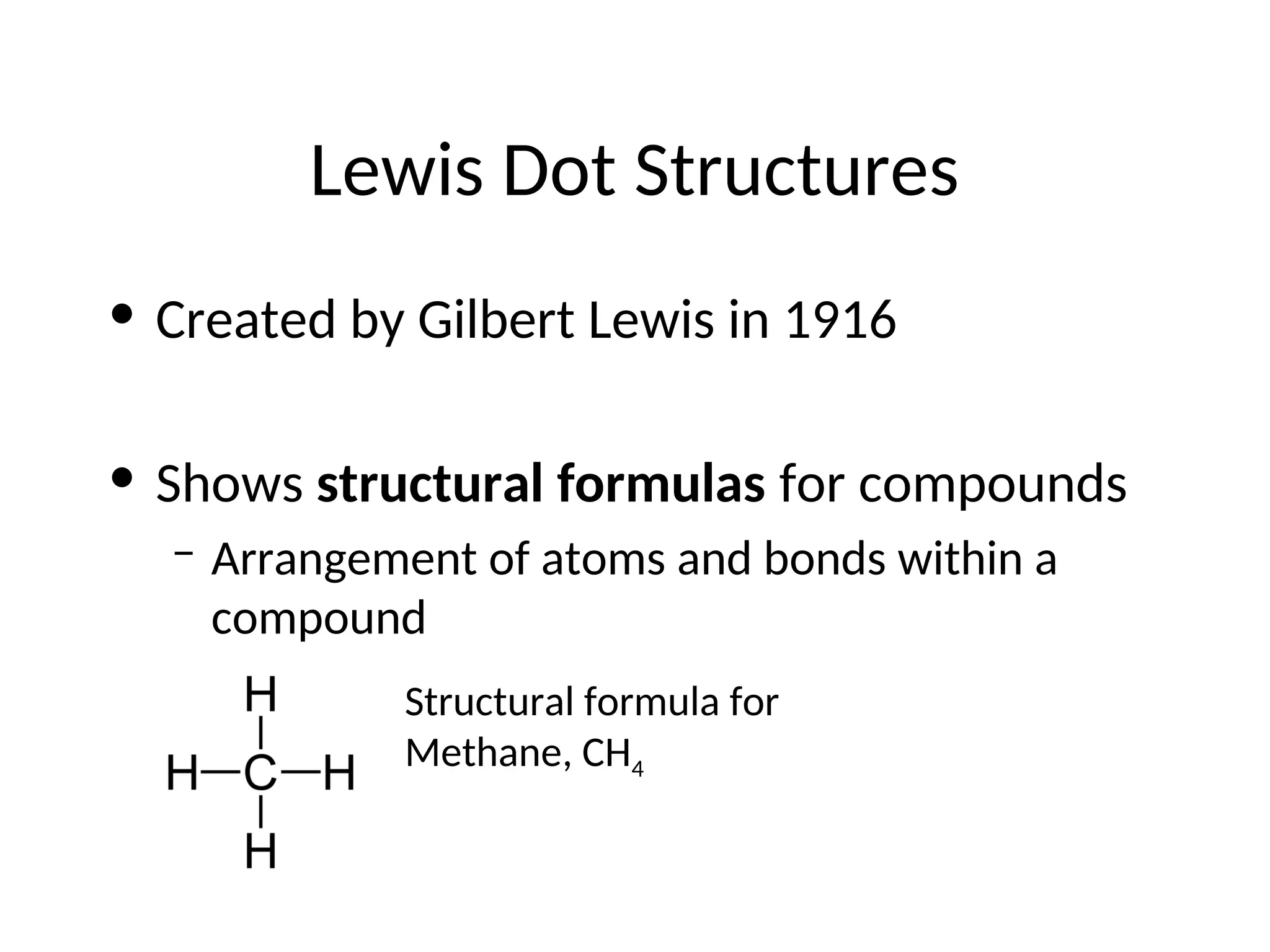
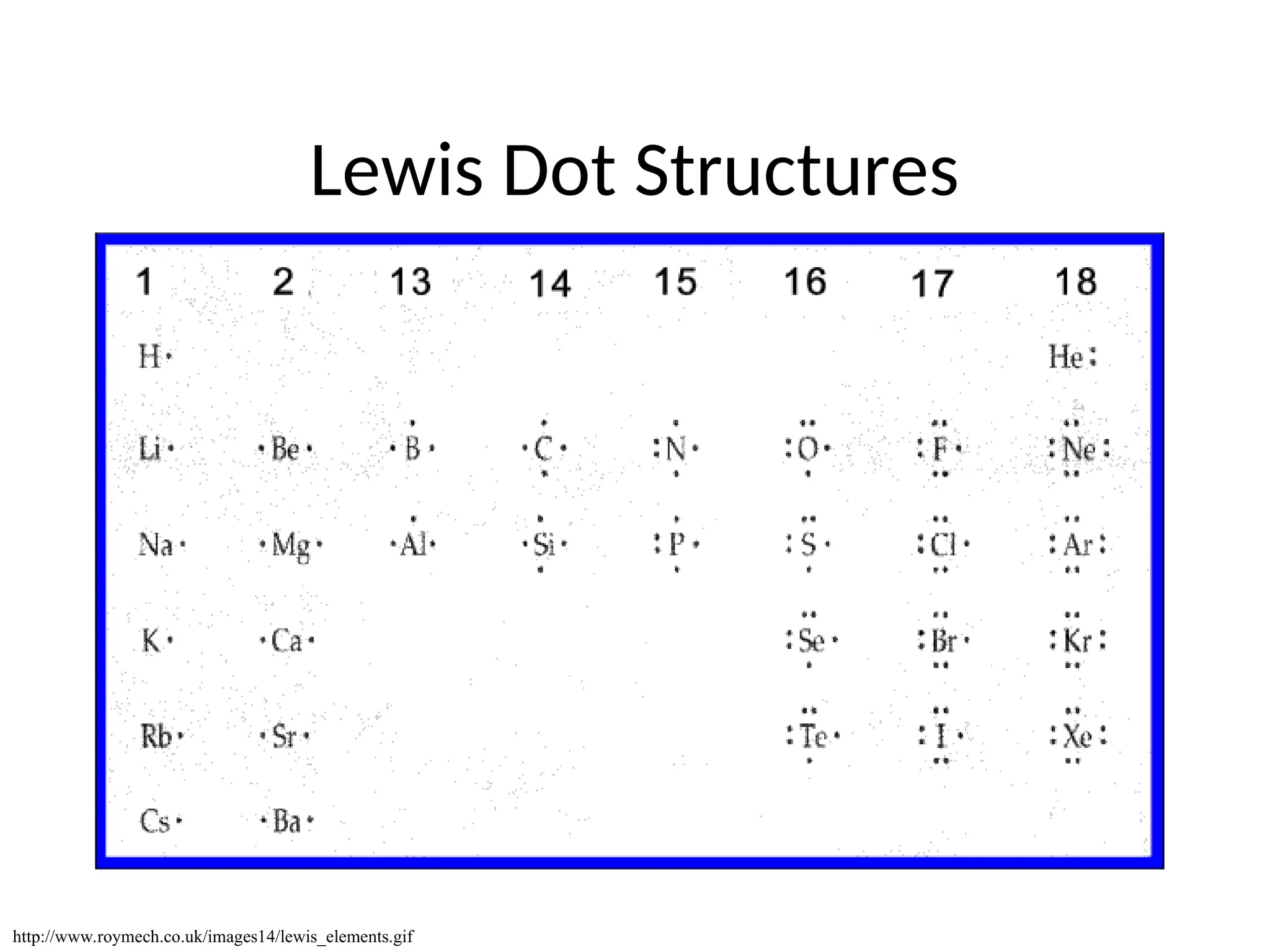
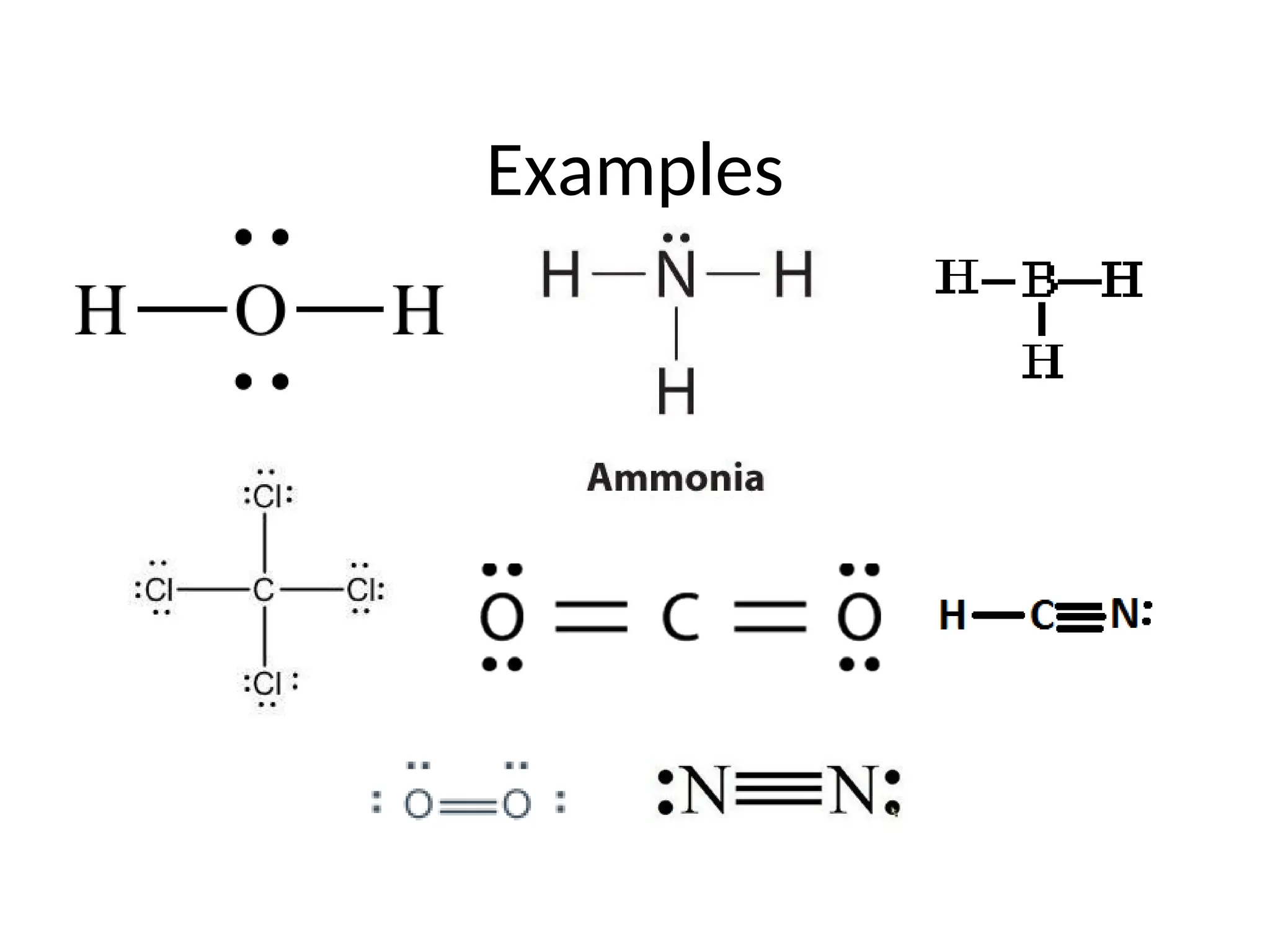
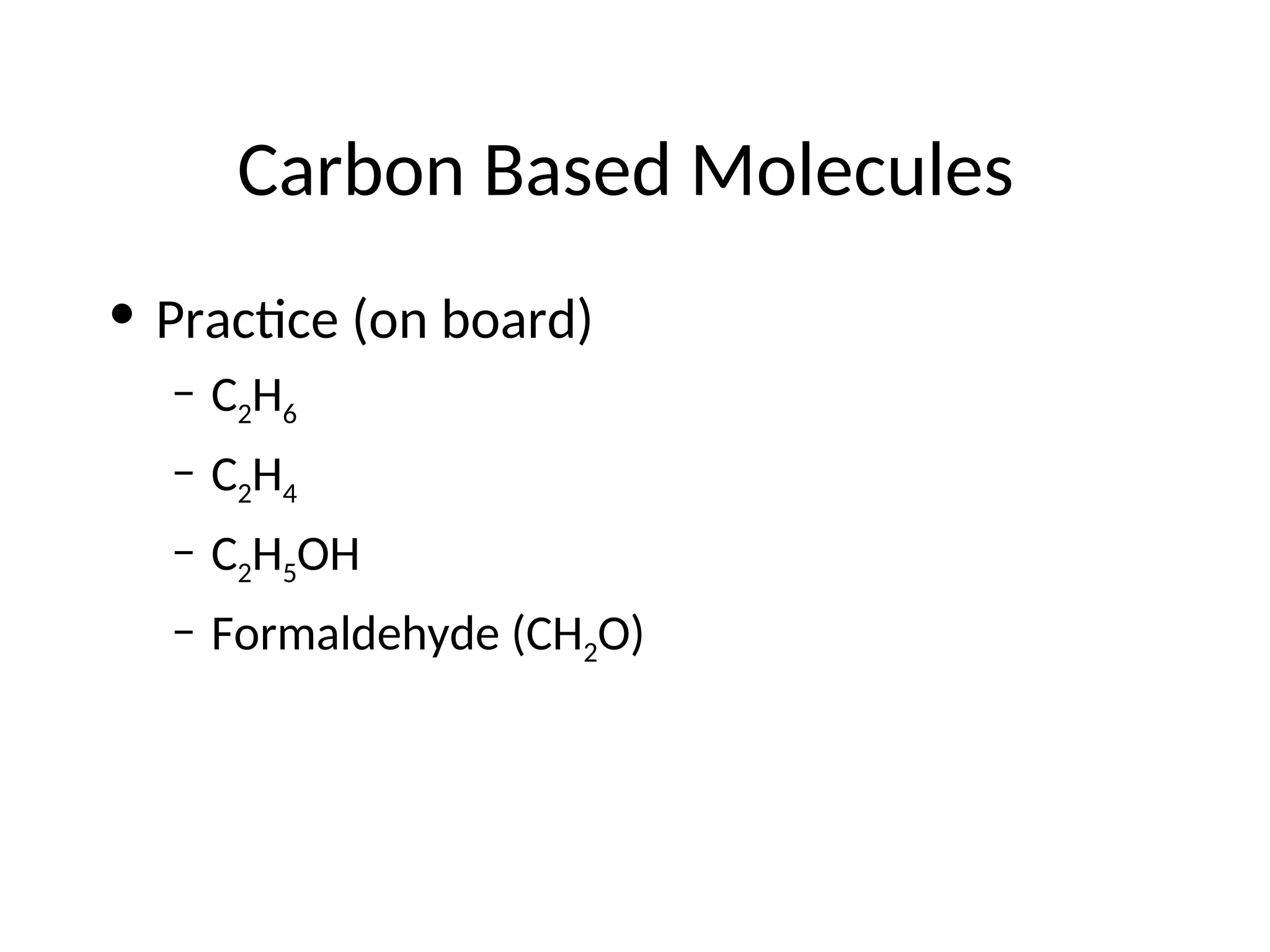
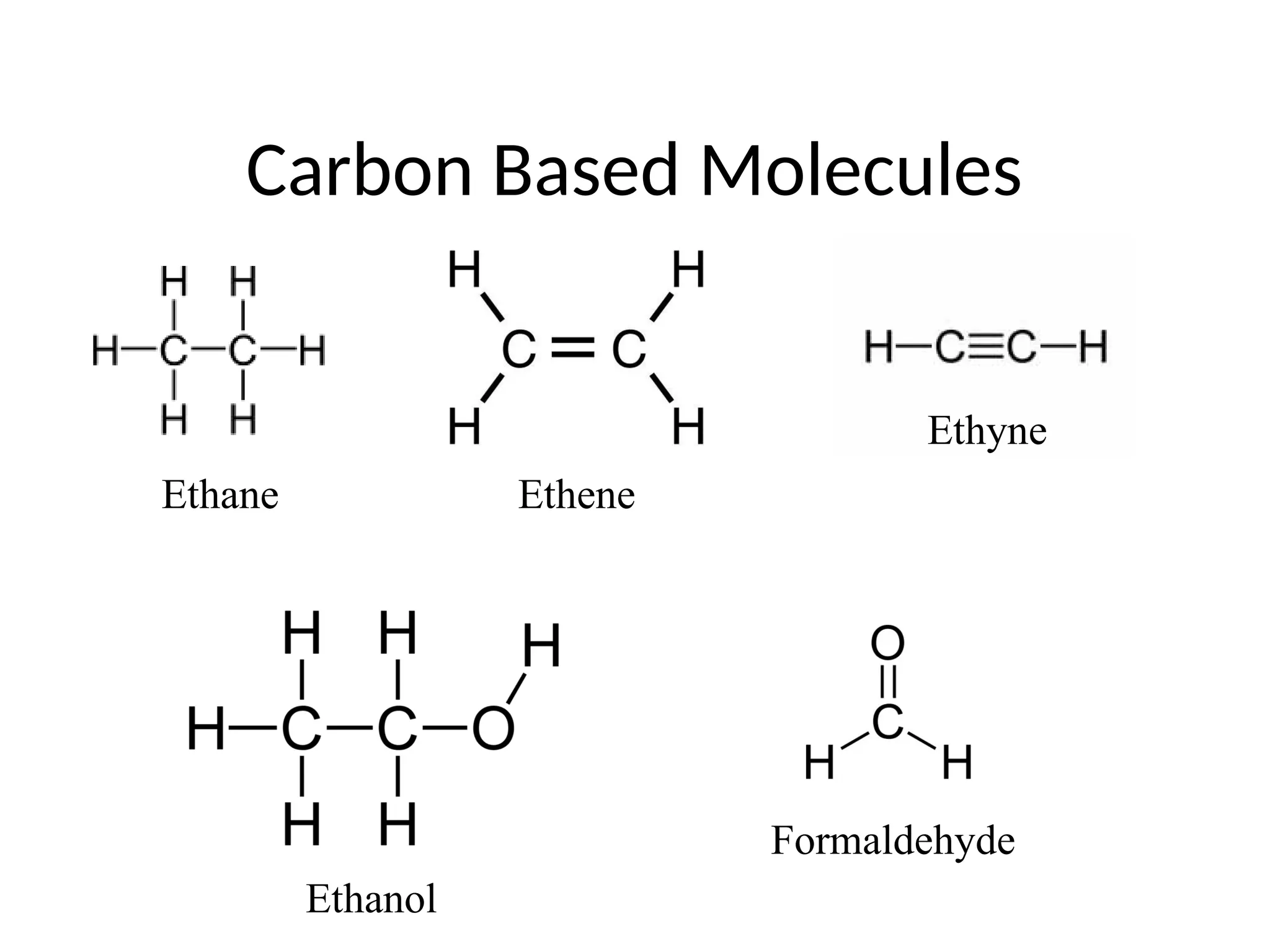
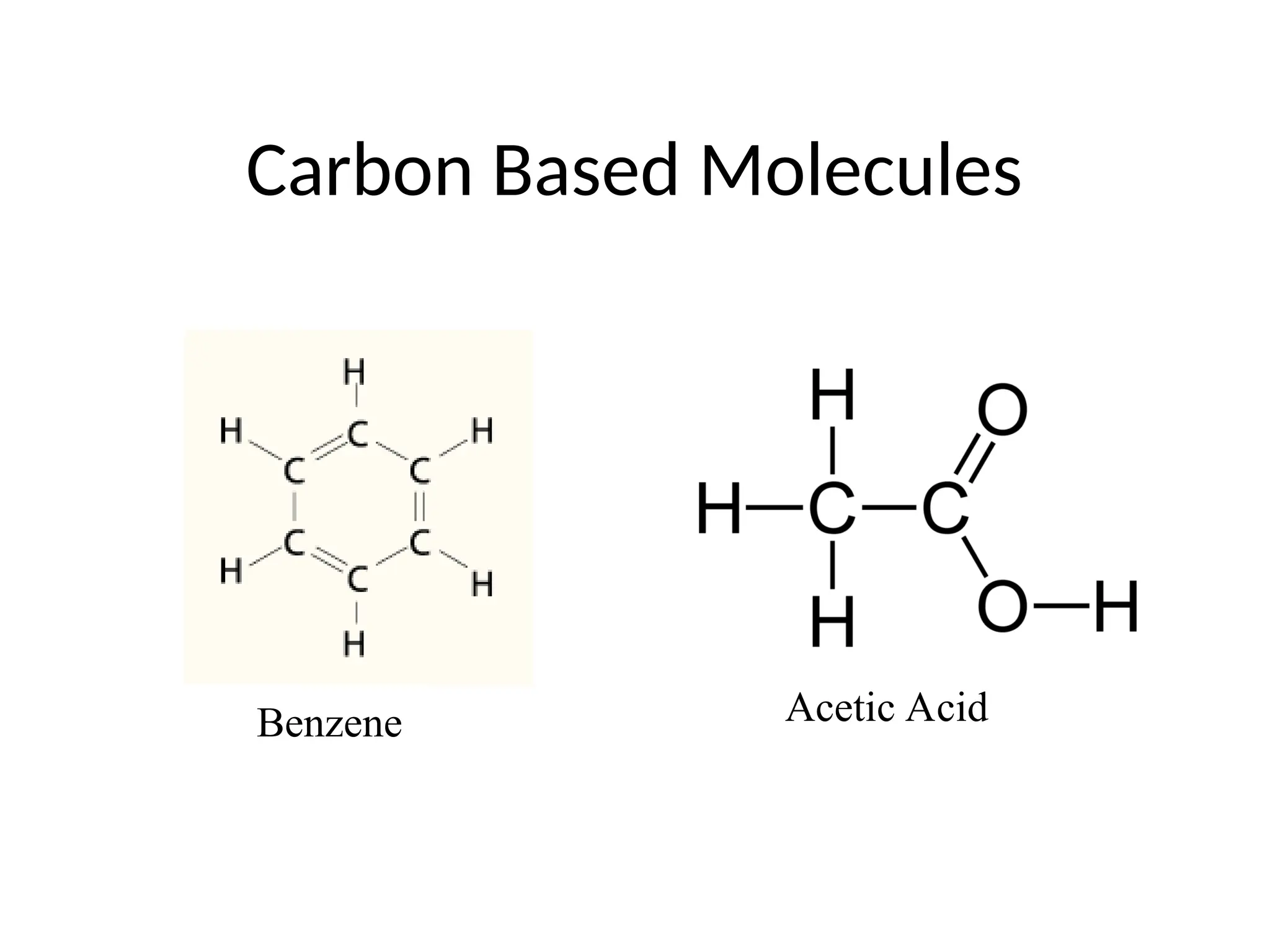
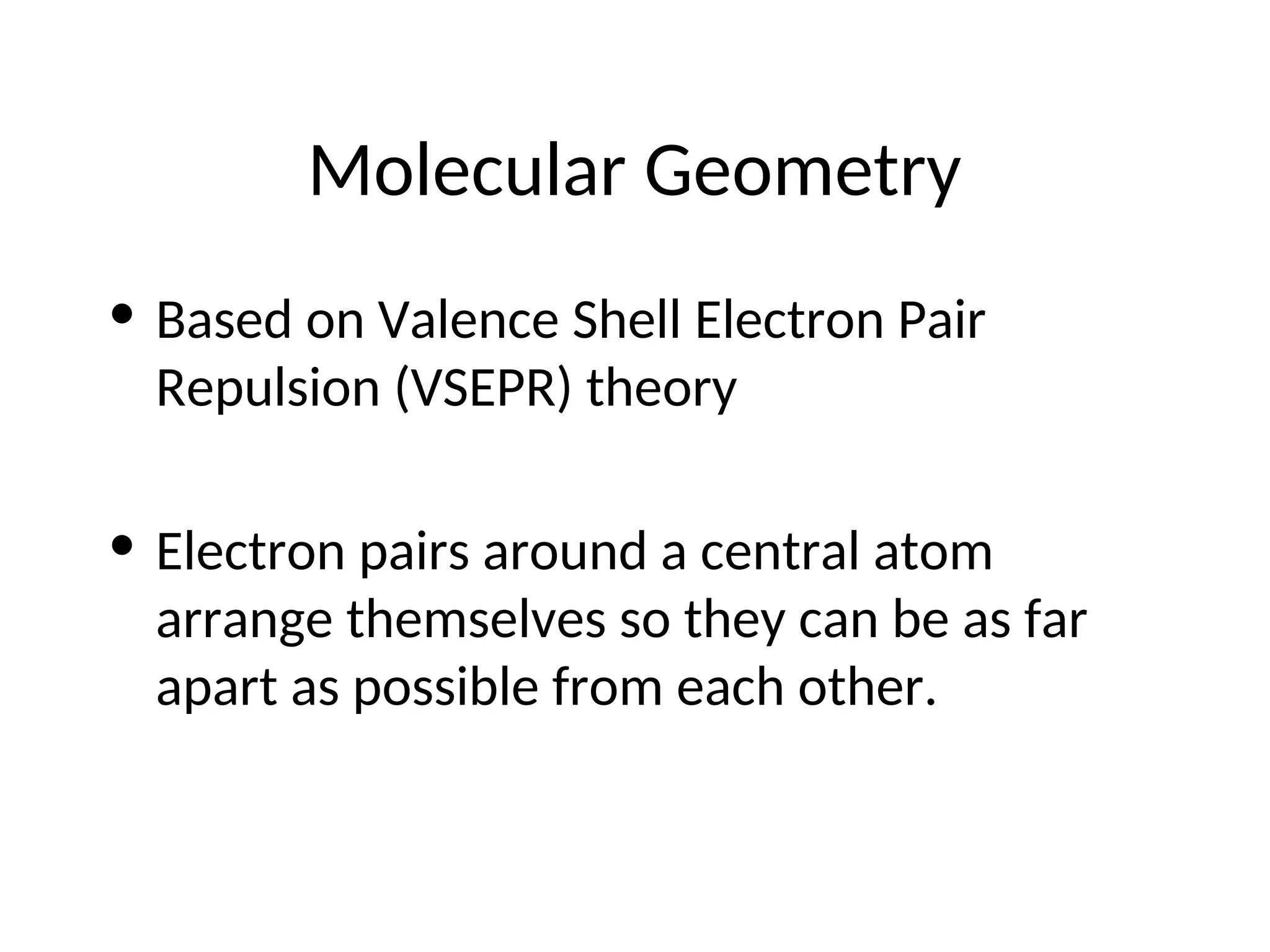
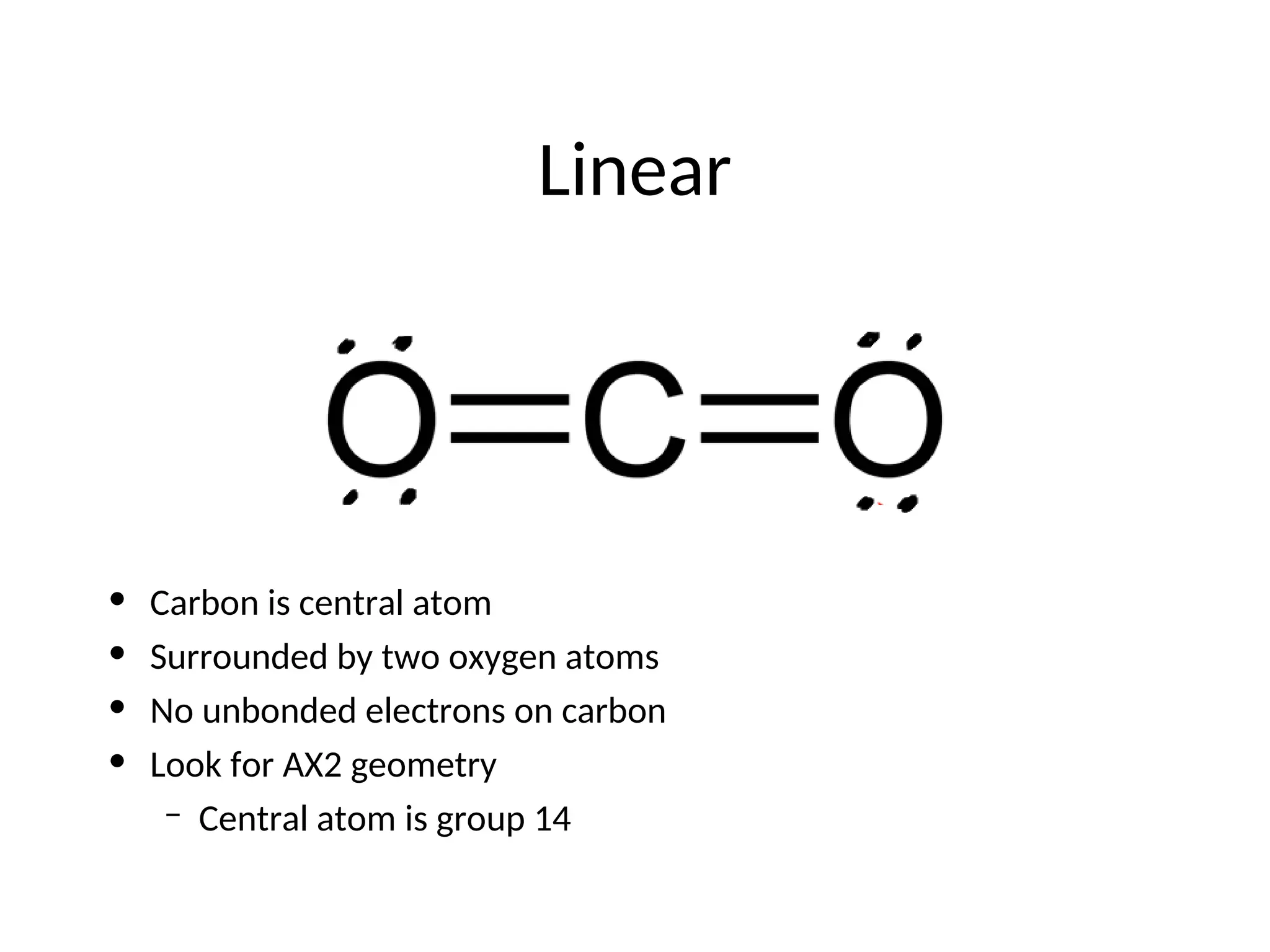
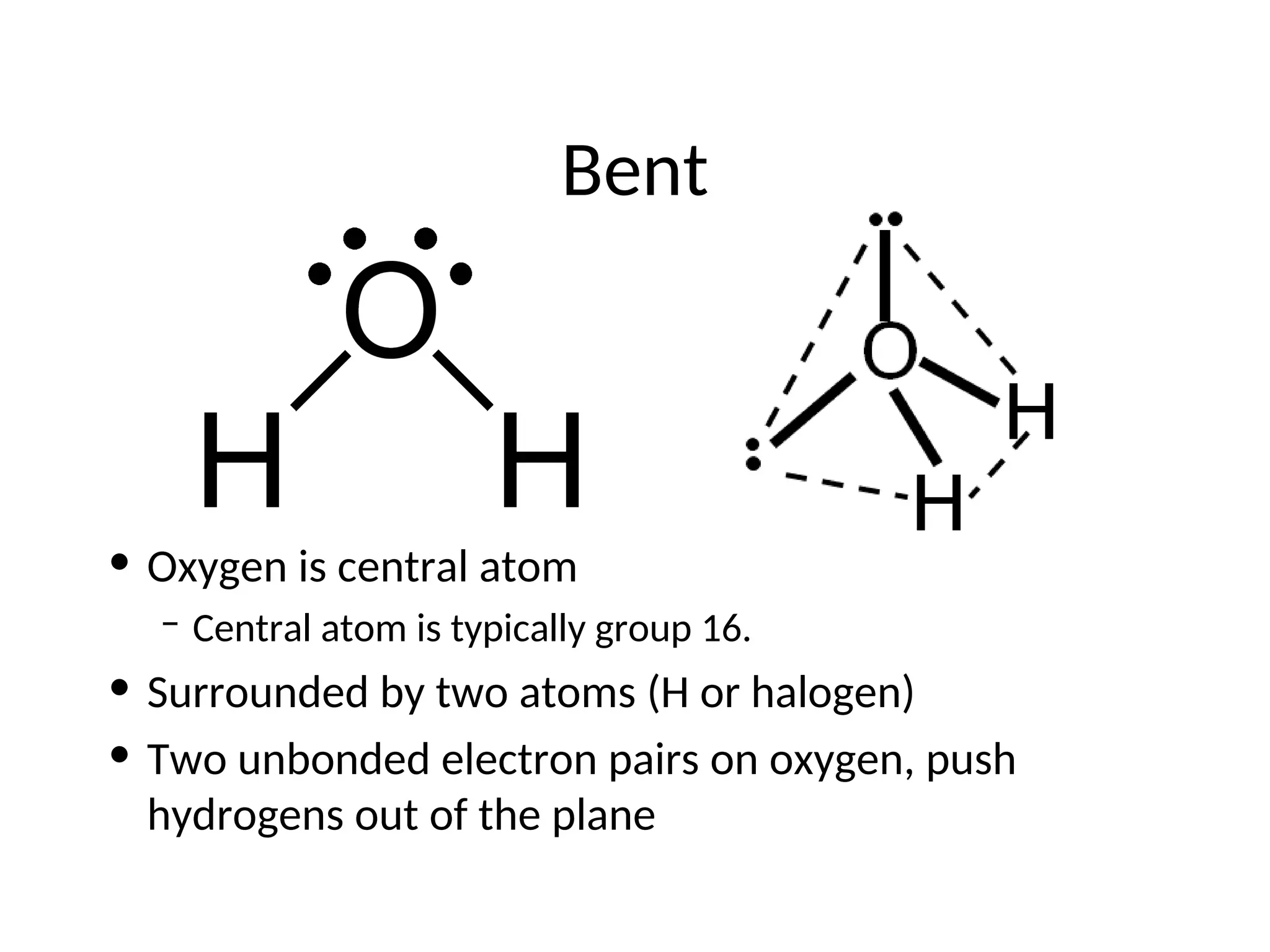
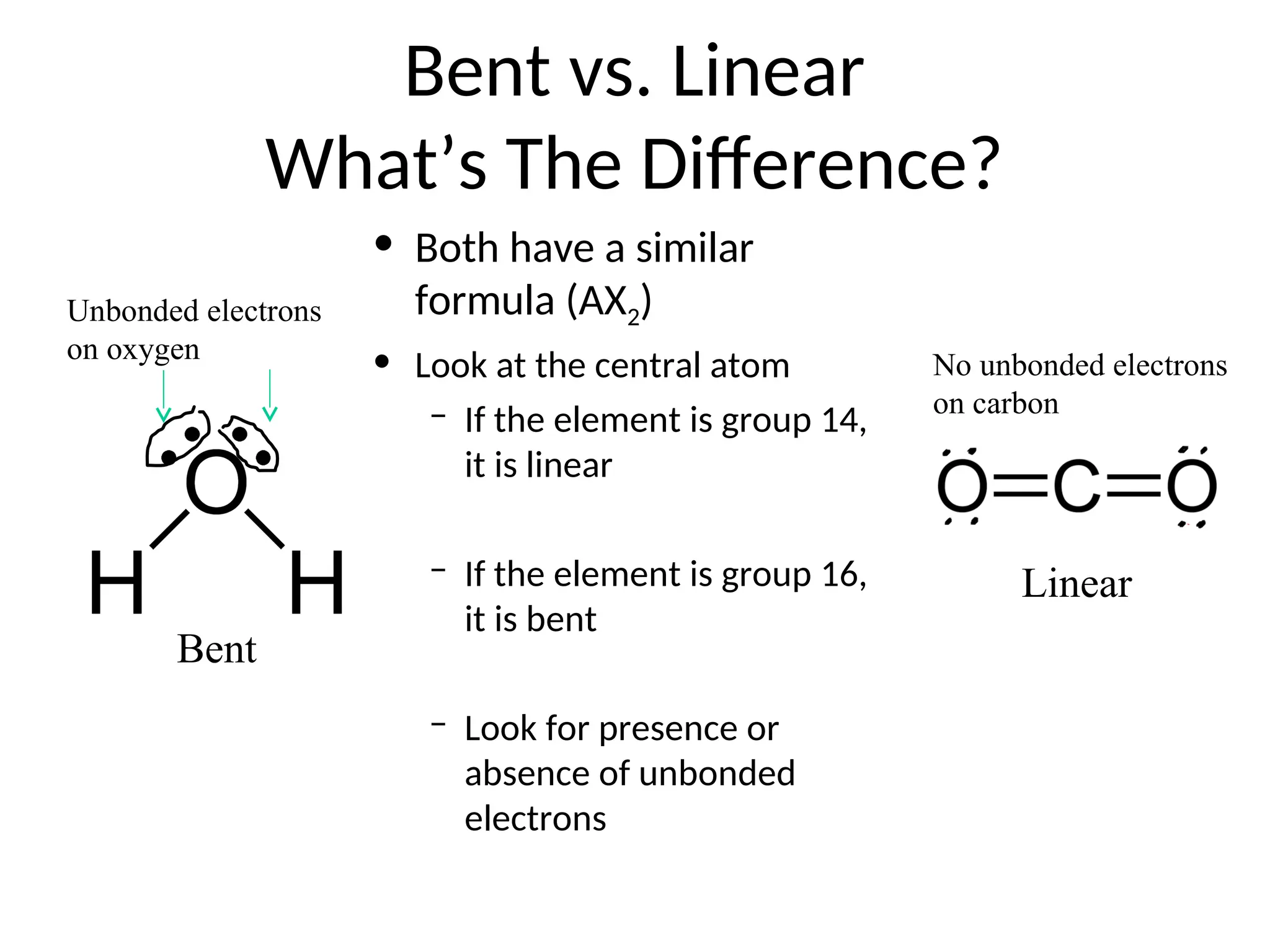
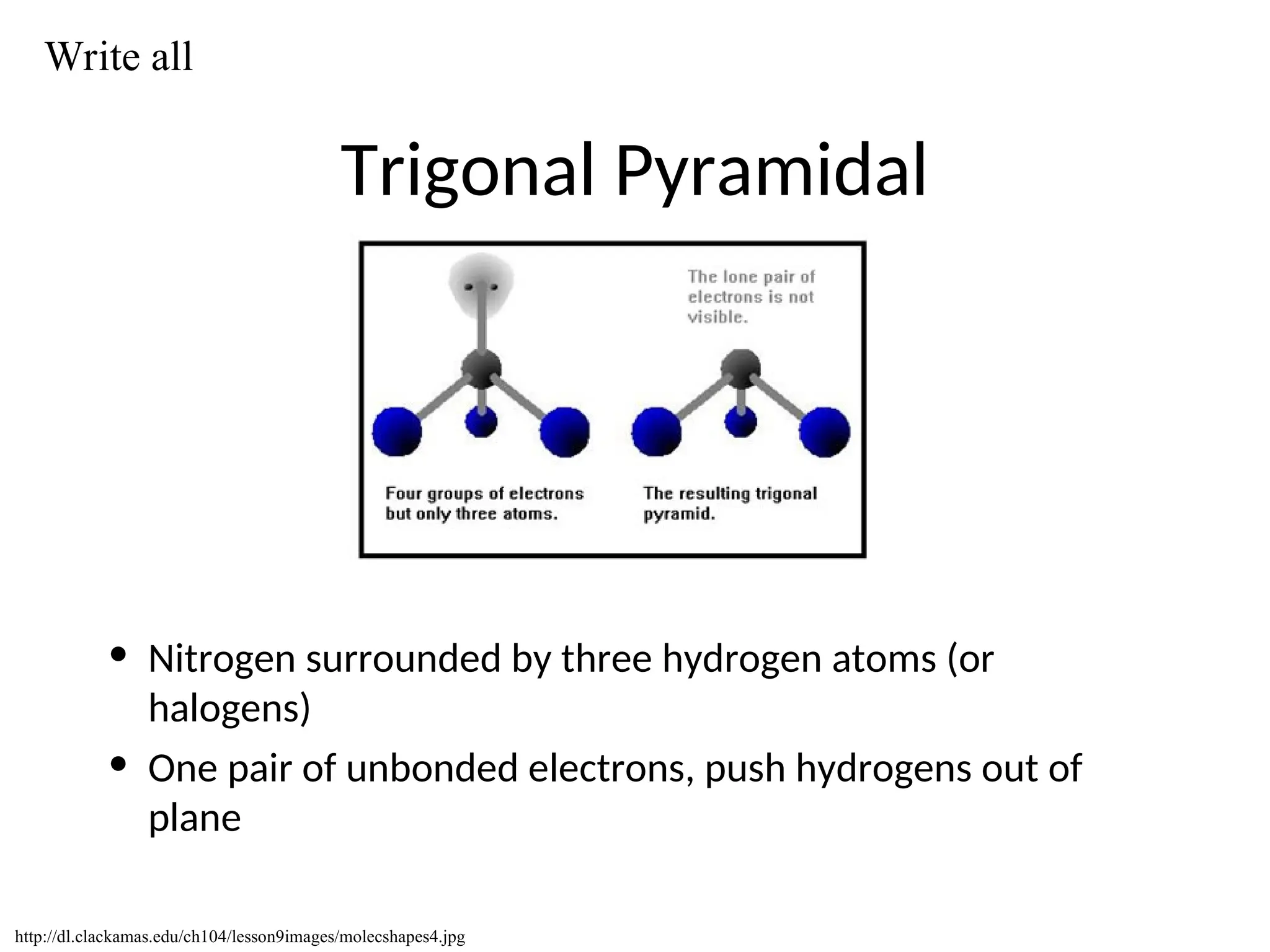
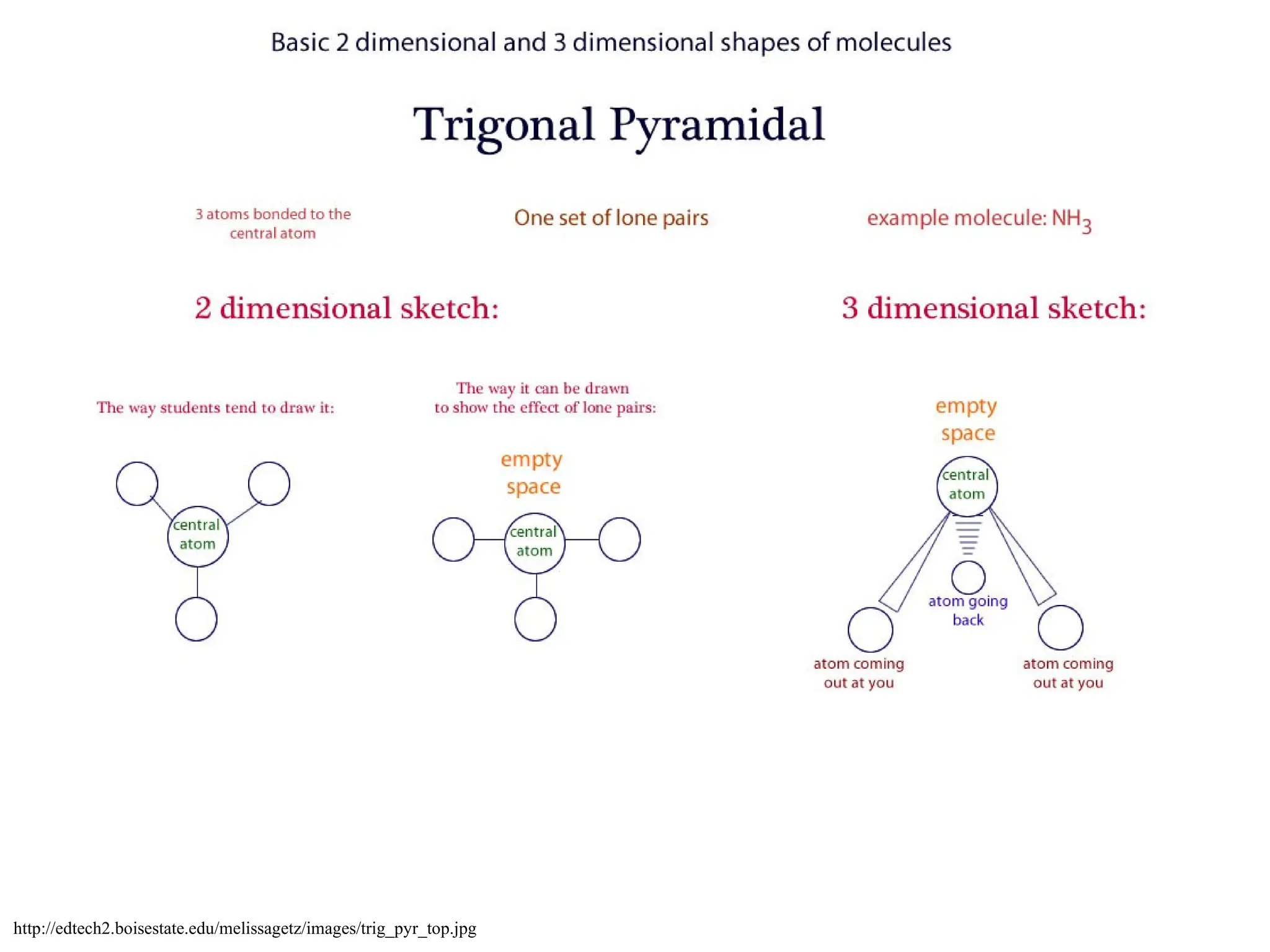
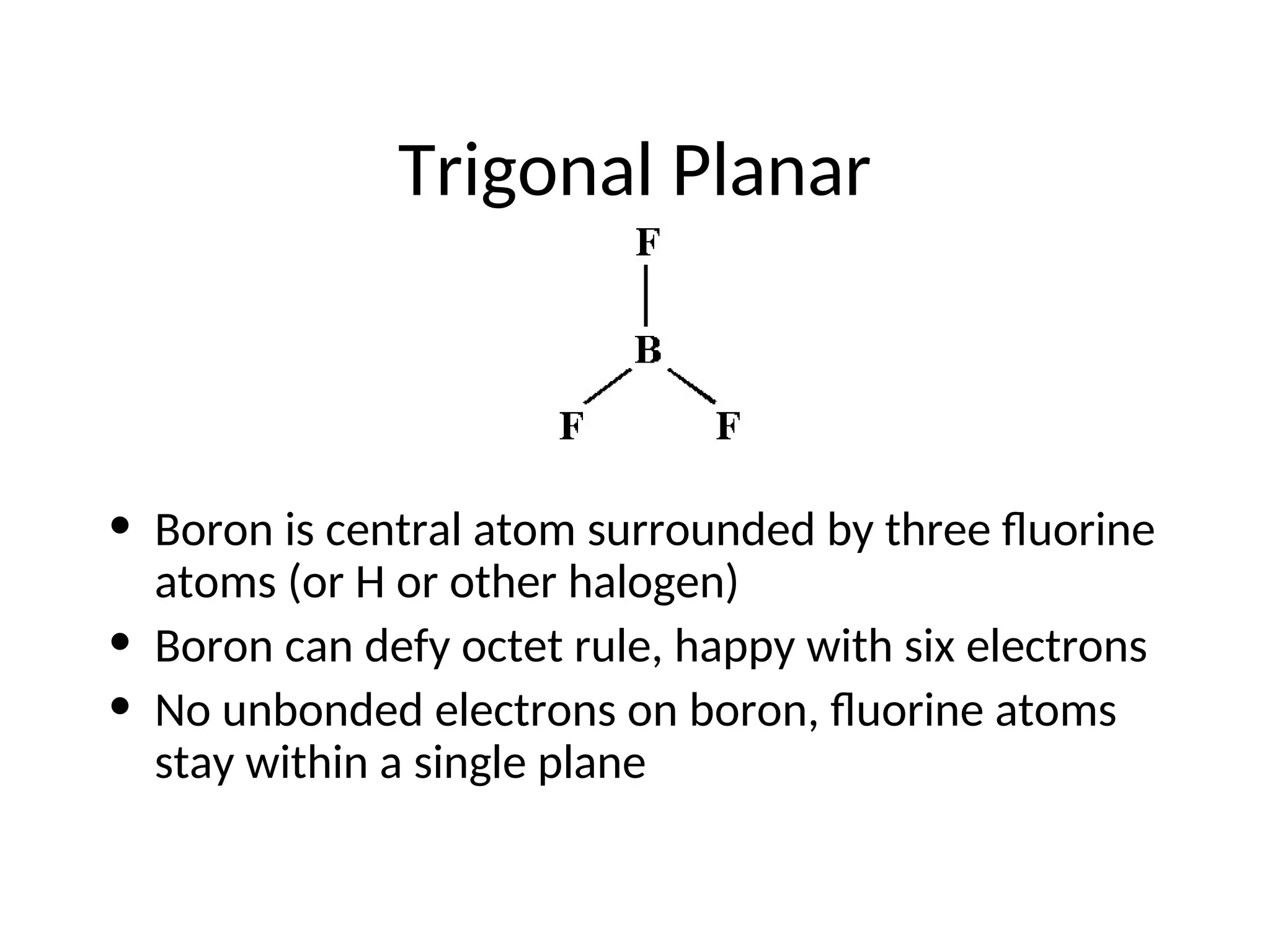
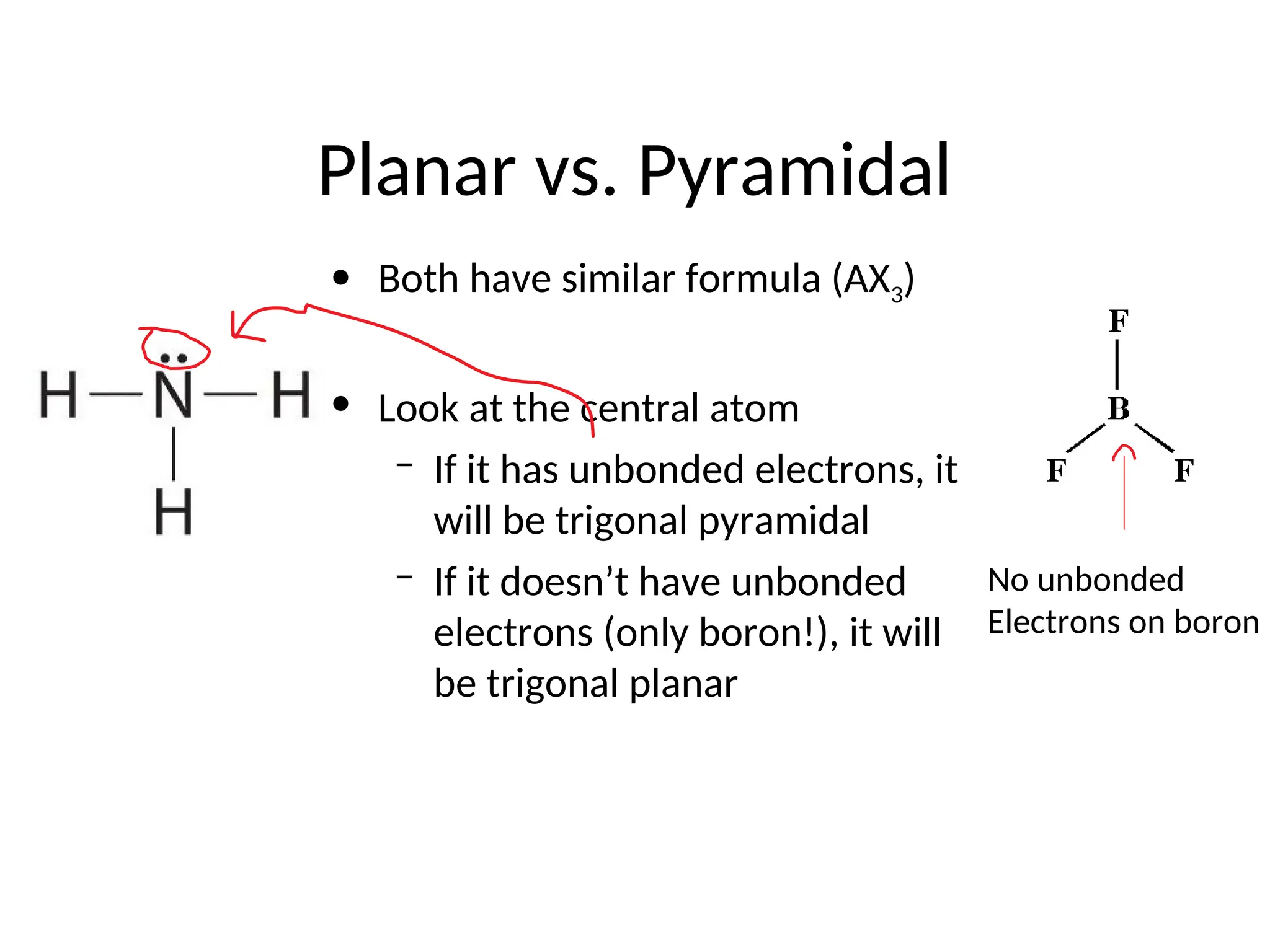
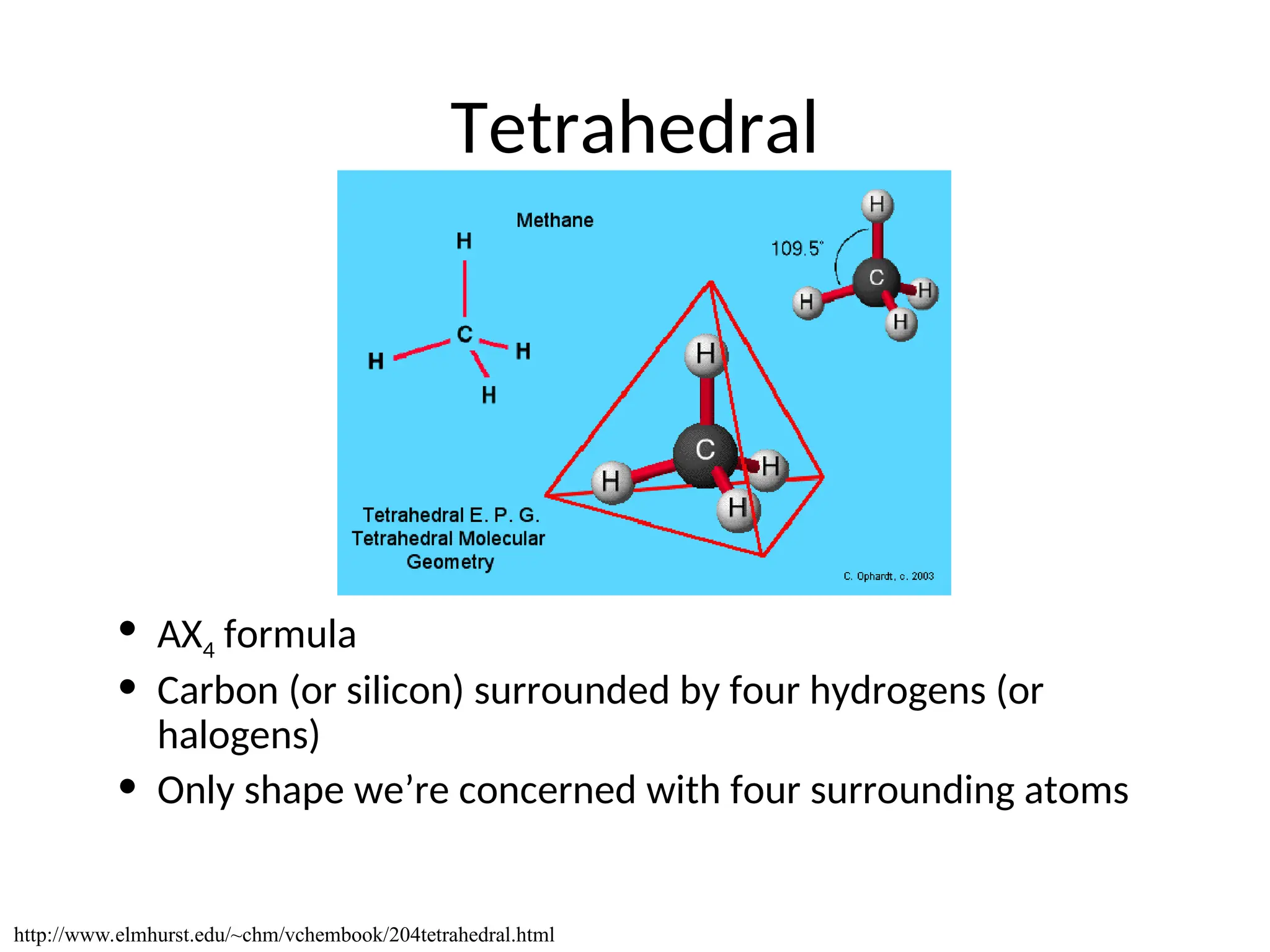
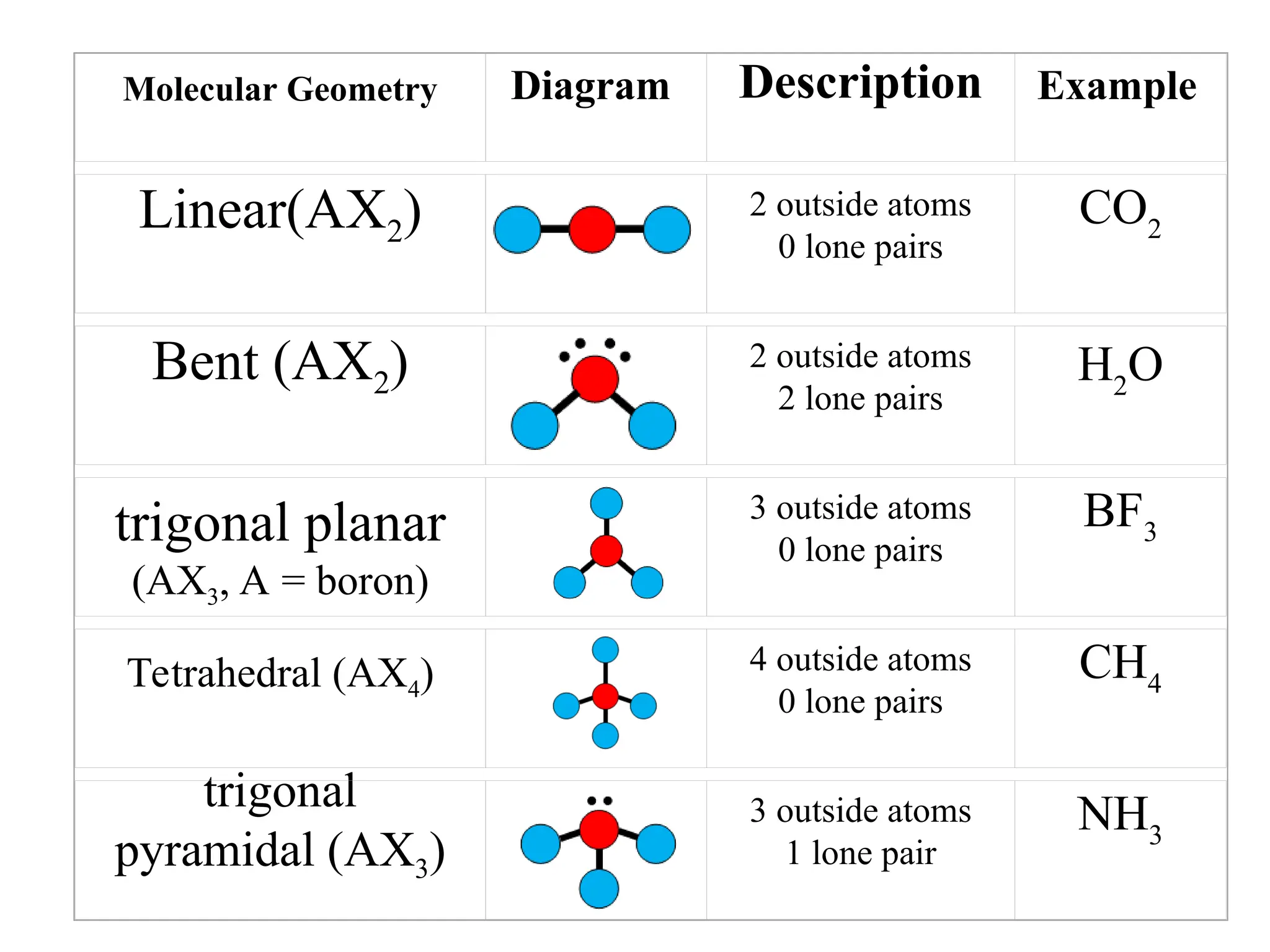
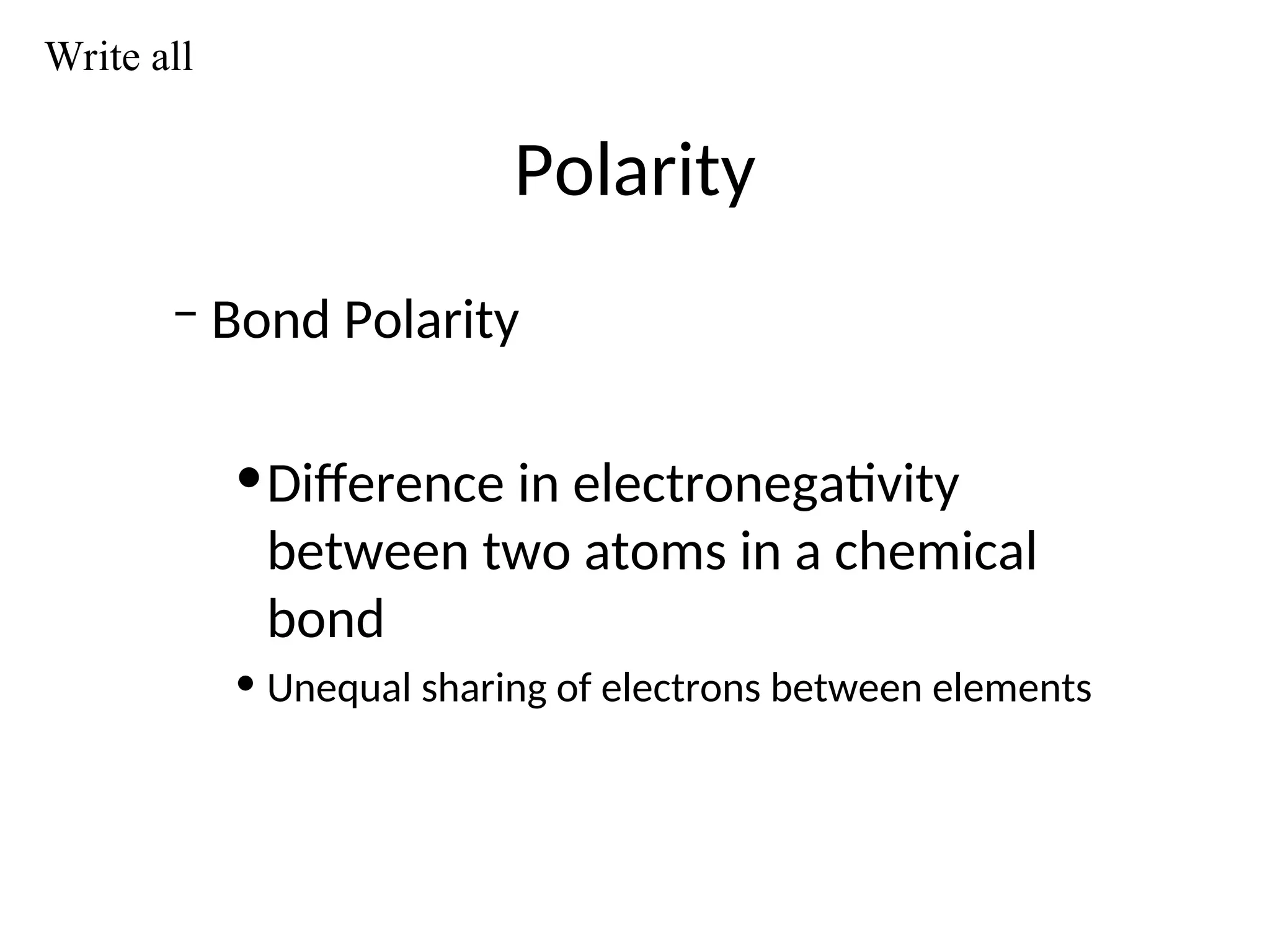
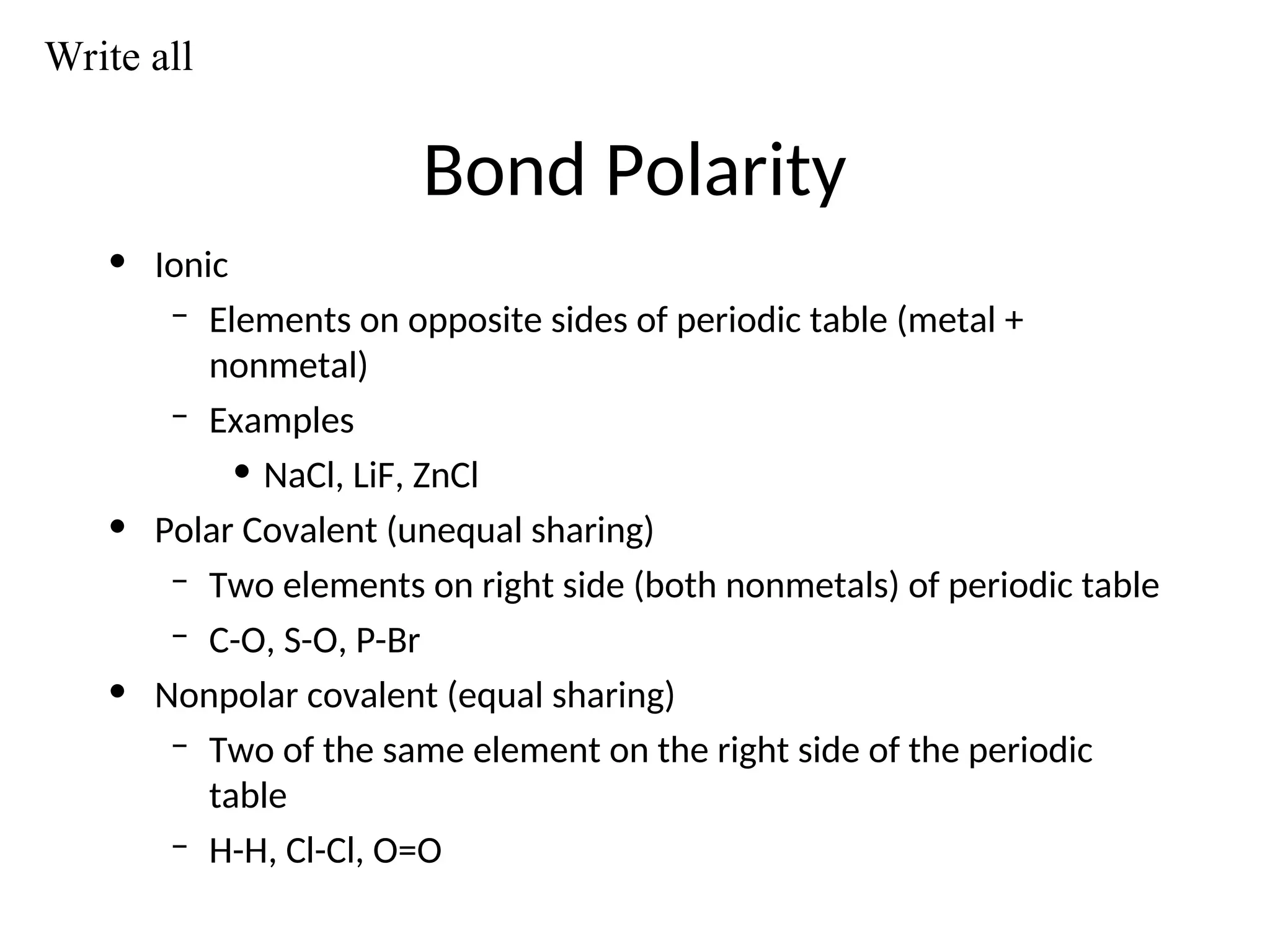
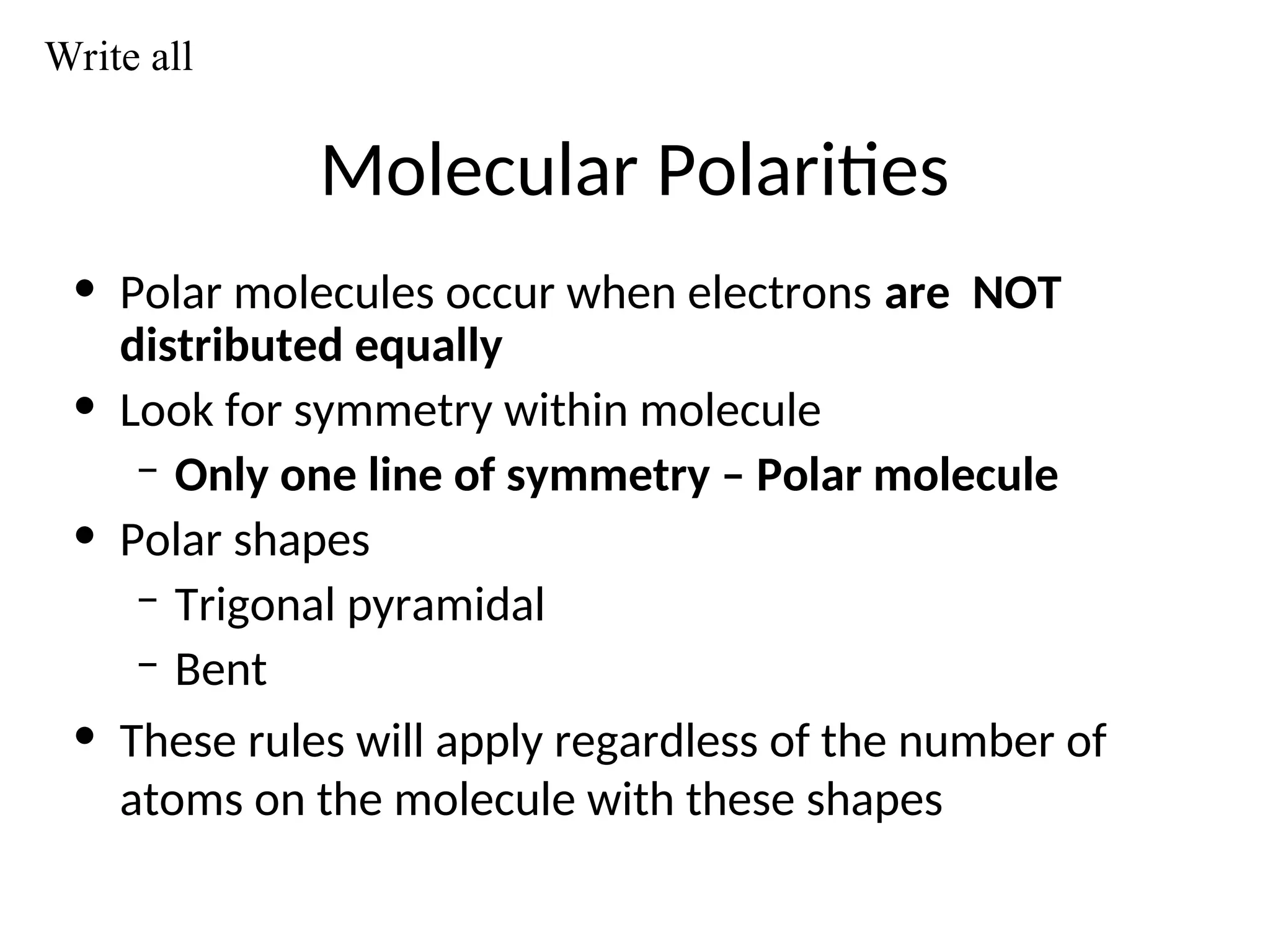
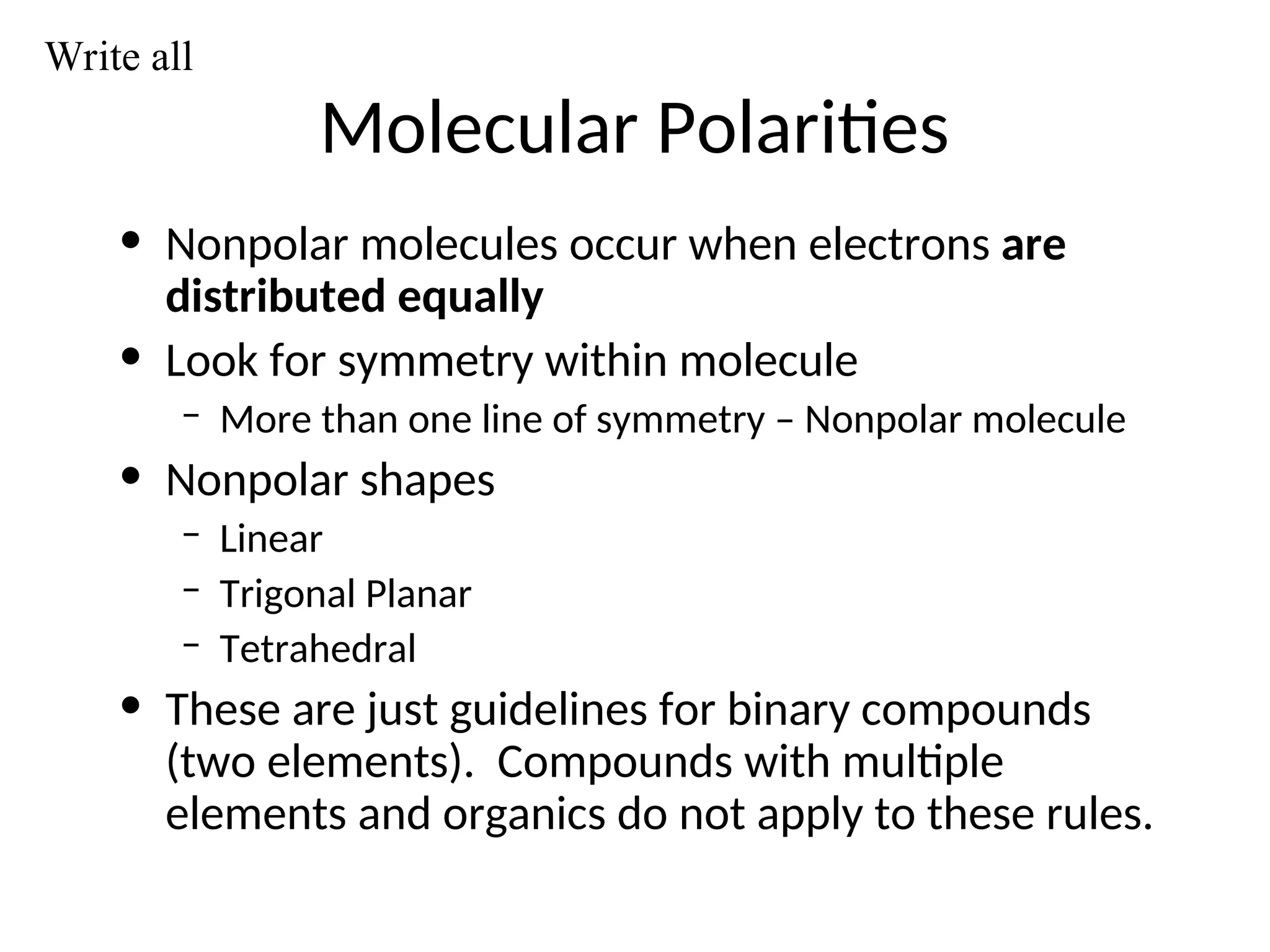
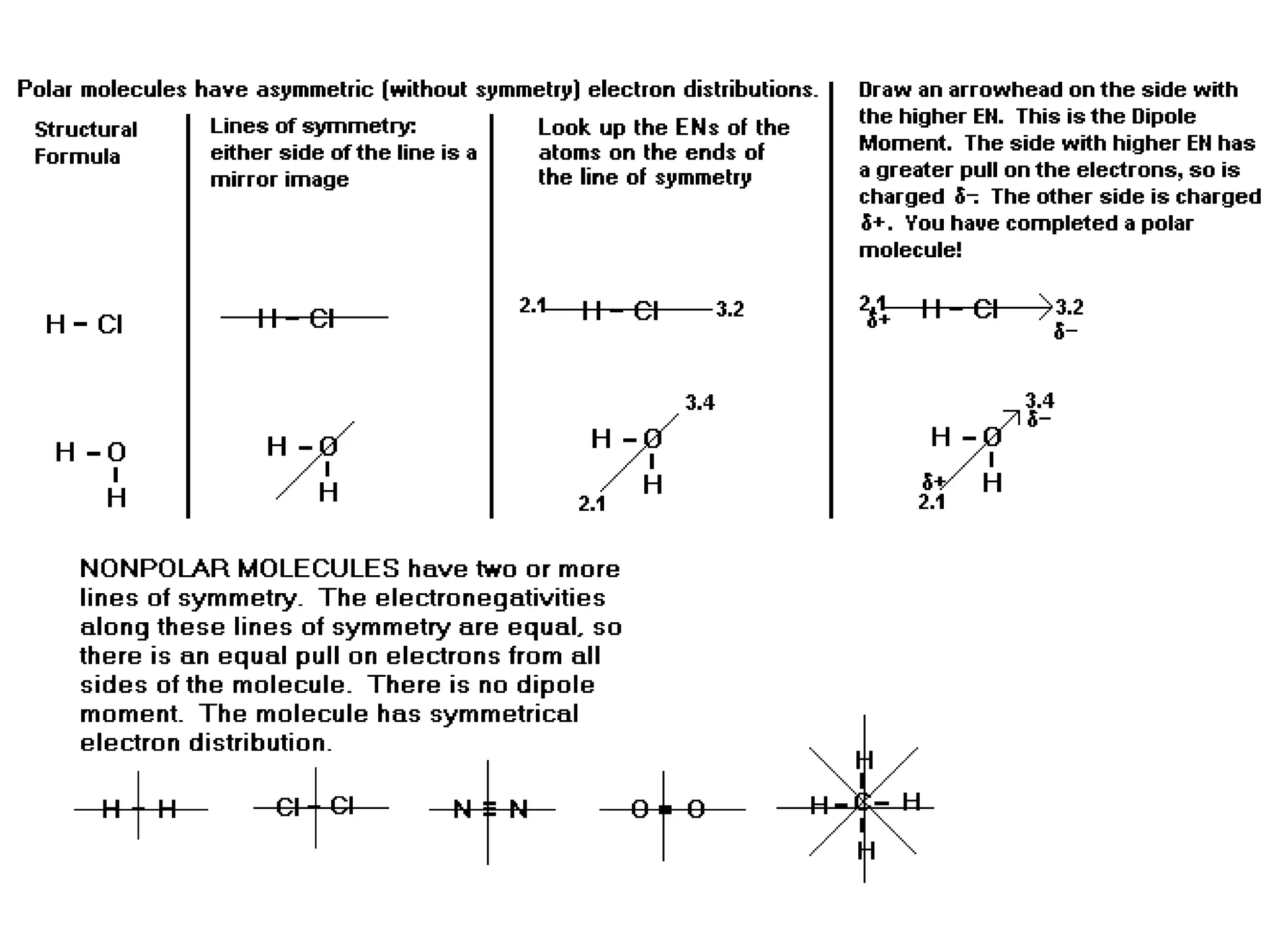
The document discusses Lewis dot structures and molecular geometries, detailing how to represent valence electrons and the arrangement of atoms in molecules. It explains the use of the Valence Shell Electron Pair Repulsion (VSEPR) model to determine molecular shapes and the concepts of bond polarity based on electronegativity. The document also provides practice exercises and emphasizes skills to master, such as drawing Lewis structures, identifying molecular shapes, and determining polarities.