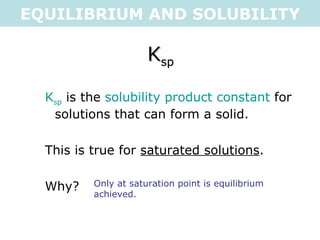
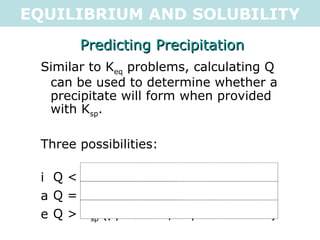
This document discusses solubility equilibria and the formation of precipitates. It defines key terms like solubility product constant (Ksp), explains how to calculate Ksp values from molar solubility and vice versa, and shows examples of using Ksp to determine whether precipitates will form when solutions are mixed. The key points are that solubility is dependent on equilibrium, saturated solutions have concentrations where Ksp = Q, and precipitates form when mixing produces Q > Ksp (supersaturation).


![EQUILIBRIUM AND SOLUBILITY Example #1 Determine the K sp expressions for the following equilibria: Ag 2 CrO 4(s) <===> 2 Ag + (aq) + CrO 4 2- (aq) BaCrO 4(s) <===> Ba 2+ (aq) + CrO 4 2- (aq) Ag 3 PO 4(s) <===> 3 Ag + (aq) + PO 4 3- (aq) K sp = [Ag + (aq) ] 2 [CrO 4 2- (aq) ] K sp = [Ba 2+ (aq) ][CrO 4 2- (aq) ] K sp = [Ag + (aq) ] 3 [PO 4 3- (aq) ]](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-4-320.jpg)


![EQUILIBRIUM AND SOLUBILITY Example #2 Silver bromide, AgBr, is the light sensitive compound in nearly all photographic film. At 25°C, one litre of water can dissolve 7.1x10 -7 mol of AgBr. Calculate the K sp of AgBr at 25°C. AgBr (s) <=> Ag + (aq) + Br - (aq) I 0 0 C +7.1x10 -7 +7.1x10 -7 E 7.1x10 -7 7.1x10 -7 Changes when it dissolves, but it is a solid. K sp = [7.1x10 -7 ][7.1x10 -7 ] K sp = 5.0x10 -13 .: K sp = 5.0x10 -13](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-7-320.jpg)
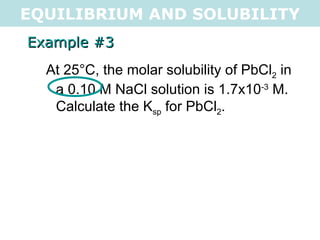
![EQUILIBRIUM AND SOLUBILITY Example #3 At 25°C, the molar solubility of PbCl 2 in a 0.10 M NaCl solution is 1.7x10 -3 M. Calculate the K sp for PbCl 2 . PbCl 2(s) <=> Pb 2+ (aq) + 2Cl - (aq) I 0 0.1 C +1.7x10 -3 +2(1.7x10 -3 ) E 1.7x10 -3 0.1034 NOTE: 0.1M Na + does not affect equilibrium K sp = [1.7x10 -3 ][0.1034] 2 K sp = 1.8x10 -5 .: K sp = 1.8x10 -5](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-9-320.jpg)
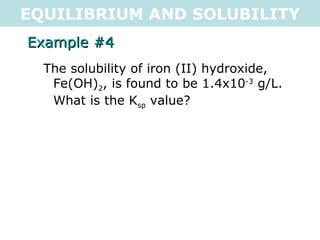
![EQUILIBRIUM AND SOLUBILITY Example #4 The solubility of iron (II) hydroxide, Fe(OH) 2 , is found to be 1.4x10 -3 g/L. What is the K sp value? n = m M = (1.4x10 -3 g) (89.861g/mol) = 1.557961741x10 -5 mol Fe(OH) 2(s) <=> Fe 2+ (aq) + 2OH - (aq) I 0 0 C +1.55796x10 -5 +2(1.55796x10 -5 ) E 1.55796x10 -5 3.11592x10 -5 K sp = [1.55796x10 -5 ][3.11592x10 -5 ] 2 K sp = 1.5x10 -14 .: K sp = 1.5x10 -14](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-11-320.jpg)

![EQUILIBRIUM AND SOLUBILITY Example #5 What is the molar solubility of AgCl in pure water at 25°C when K sp = 1.8x10 -10 . AgCl (s) <=> Ag + (aq) + Cl - (aq) I 0 0 C +x +x E x x K sp = [x][x] K sp = x 2 1.8x10 -10 = x 2 1.34x10 -5 M = x .: the molar solubility is 1.34x10 -5 mol/L](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-13-320.jpg)

![EQUILIBRIUM AND SOLUBILITY Example #6 The K sp for magnesium fluoride, MgF 2 , has a value of 6.4x10 -9 . What is its solubility in g/L? MgF 2(s) <=> Mg 2+ (aq) + 2F - (aq) I 0 0 C +x +2x E x 2x K sp = [x][2x] 2 = 6.4x10 -9 4x 3 = 6.4x10 -9 x = 6.4x10 -9 4 x = 1.16x10 -3 mol/L .: the solubility is 7.2x10 -2 g/L 3](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-15-320.jpg)
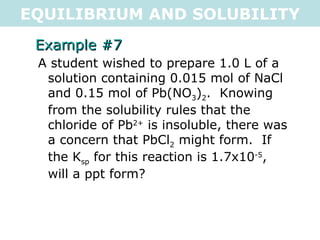
![EQUILIBRIUM AND SOLUBILITY Example #7 A student wished to prepare 1.0 L of a solution containing 0.015 mol of NaCl and 0.15 mol of Pb(NO 3 ) 2 . Knowing from the solubility rules that the chloride of Pb 2+ is insoluble, there was a concern that PbCl 2 might form. If the K sp for this reaction is 1.7x10 -5 , will a ppt form? PbCl 2(s) <=> Pb 2+ (aq) + 2Cl - (aq) 0.15 0.015 K sp = [Pb 2+ (aq) ][Cl - (aq) ] 2 Q = [Pb 2+ (aq) ][Cl - (aq) ] 2 Q = [0.15][0.015] 2 Q = 3.375x10 -5 Q > K sp , so a precipitate WILL form .: a precipitate will form](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-18-320.jpg)
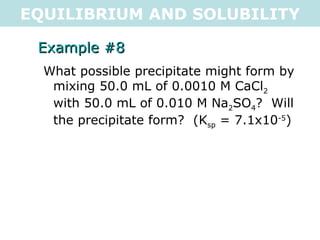
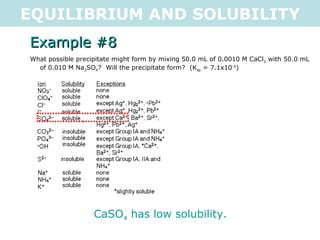
![EQUILIBRIUM AND SOLUBILITY Example #8 What possible precipitate might form by mixing 50.0 mL of 0.0010 M CaCl 2 with 50.0 mL of 0.010 M Na 2 SO 4 ? Will the precipitate form? (K sp = 7.1x10 -5 ) CaSO 4(s) <=> Ca 2+ (aq) + SO 4 2- (aq) C 1 V 1 =C 2 V 2 C 2 = C 1 V 1 V 2 C 2 = 0.0010M x 0.050L 0.100L C 2 = 5x10 -4 M C 2 = C 1 V 1 V 2 C 2 = 5x10 -3 M Q = [Ca 2+ (aq) ][SO 4 2- (aq) ] Q = [0.0005][0.005] Q = 2.5x10 -6 Q < K sp , so a precipitate will NOT form (unsaturated) .: the possible precipitate, CaSO 4 , will not form](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-21-320.jpg)
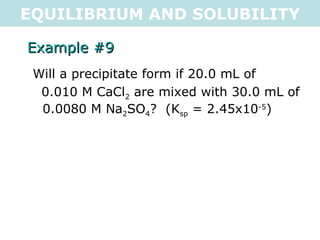
![EQUILIBRIUM AND SOLUBILITY Example #9 Will a precipitate form if 20.0 mL of 0.010 M CaCl 2 are mixed with 30.0 mL of 0.0080 M Na 2 SO 4 ? (K sp = 2.45x10 -5 ) CaSO 4(s) <=> Ca 2+ (aq) + SO 4 2- (aq) C 2 = C 1 V 1 V 2 C 2 = 0.010M x 0.020L 0.050L C 2 = 4x10 -3 M C 2 = C 1 V 1 V 2 C 2 = 0.0080M x 0.030L 0.050L C 2 = 4.8x10 -3 M Q = [Ca 2+ (aq) ][SO 4 2- (aq) ] Q = [0.004][0.0048] Q = 1.92x10 -5 Q < K sp , so a precipitate will NOT form (unsaturated) .: a precipitate will not form](https://image.slidesharecdn.com/tang07-equilibriumandsolubility2-111017141233-phpapp02/85/Tang-07-equilibrium-and-solubility-2-23-320.jpg)