- Contrast media are substances used in medical imaging to increase radiographic contrast in areas where it was previously low or absent. They improve the visibility of internal structures on scans.
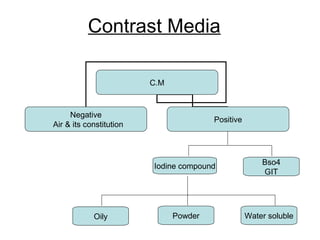
- There are two main types - positive contrast agents, which increase contrast, and negative contrast agents, which decrease contrast. Common positive agents are iodine-based and barium-based. Common negative agents are air and carbon dioxide.
- Contrast media are administered in different ways depending on the area being examined, such as orally, rectally, intravenously, or intra-arterially. They allow detailed examination of organ systems like the gastrointestinal tract, blood vessels, and soft tissues.