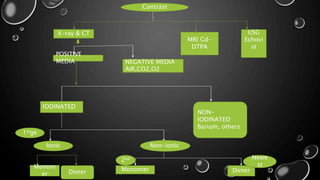
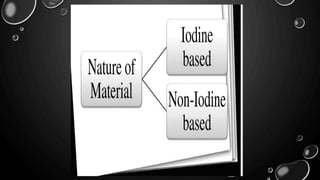
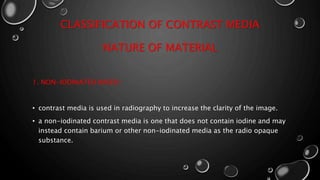
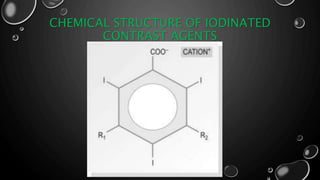
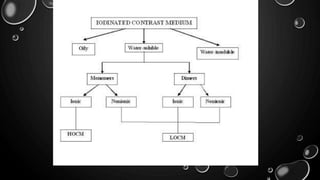
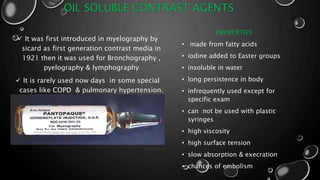
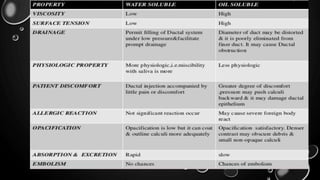
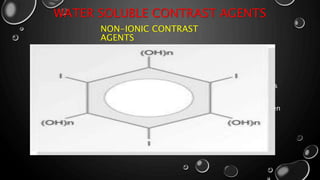
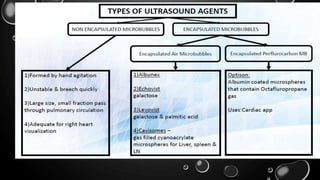
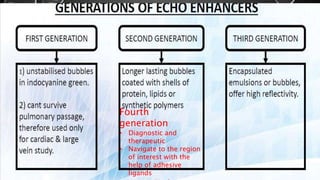
This document provides an overview of radiographic contrast media. It discusses how contrast media enhance images by increasing the absorption of x-rays in certain tissues. It describes the ideal properties of contrast media and classifications such as iodinated versus non-iodinated, ionic versus non-ionic, monomer versus dimer. Examples are given for different types of contrast media including barium sulfate, iodinated monomers and dimers, oil-soluble agents, and MRI contrast agents containing gadolinium. The document covers the history, properties, advantages, disadvantages and examples of various contrast media used in radiology.



![HISTORY OF CONTRAST MEDIA
• IN 1920S, THE FIRST RADIOGRAPHIC CONTRAST MEDIUM INTRODUCED, SODIUM
IODIDE.
• FIRST MAJOR BREAKTHROUGH - IODINE WAS BOUND TO ORGANIC MOLECULES
[ UROSELECTAN (IOPAX), UROSELECTAN-B (NEOIOPAX) ].
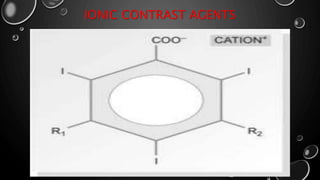
• 1960S, MAJORITY OF WATER SOLUBLE CONTRAST MEDIA WERE SALTS OF IODINATED
FULLY SUBSTITUTED BENZOIC ACID DERIVATIVES [TRIIODINATED BENZOIC ACID].
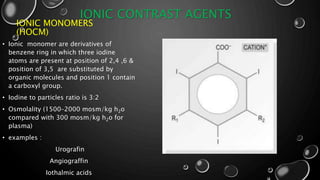
• 1970S, INTRODUCTION OF LOW OSMOLAR CONTRAST MEDIA (LOCM) - [ IOHEXOL,
IOVERSOL,IOPAMIDOL AND IOBITRIDOL ].
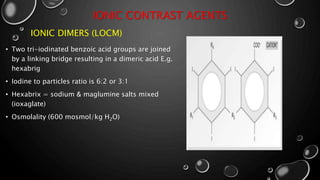
• 1980S AND 1990S, ONGOING DEVELOPMENT OF NON-IONIC ISOTONIC DIMERS.](https://image.slidesharecdn.com/dineshsapkota1st-180822053118/85/contrast-medium-5-320.jpg)