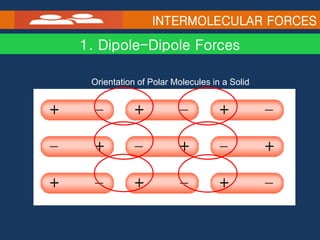
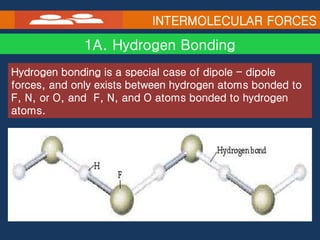
Intramolecular forces hold atoms together within a molecule, such as covalent bonds. Intermolecular forces exist between separate molecules and include dipole-dipole interactions between polar molecules, hydrogen bonding, ion-dipole interactions, and dispersion forces between all molecules due to induced dipoles. The type of intermolecular force present influences physical properties like melting and boiling points, with ionic compounds having the highest melting points due to strong ion-ion interactions.