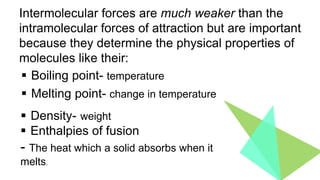
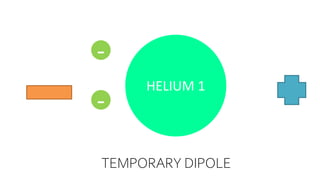
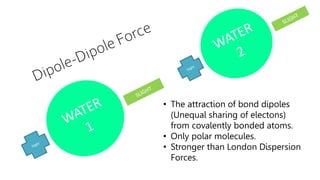
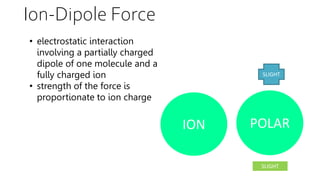
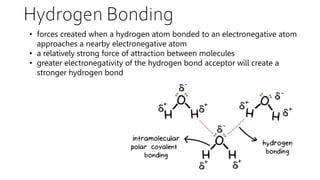
Intermolecular forces are weaker than intramolecular forces but determine properties like boiling point, melting point, and density. There are several types of intermolecular forces including London dispersion forces, dipole-dipole interactions, hydrogen bonding, and ion-dipole interactions. London dispersion forces result from temporary dipoles between all molecules and increase with atomic mass. Dipole-dipole interactions occur between polar molecules while hydrogen bonding is particularly strong between a hydrogen atom bonded to an electronegative atom like nitrogen, oxygen, or fluorine. Stronger intermolecular forces require more energy to overcome.