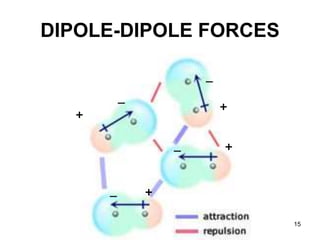
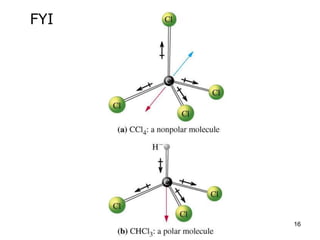
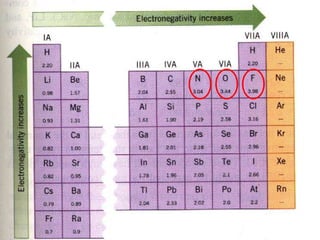
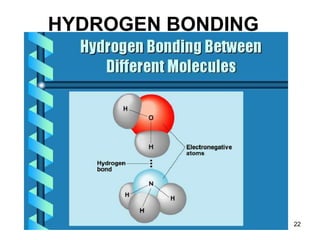
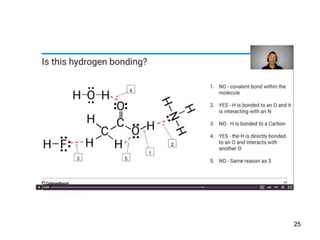
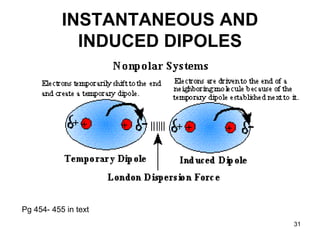
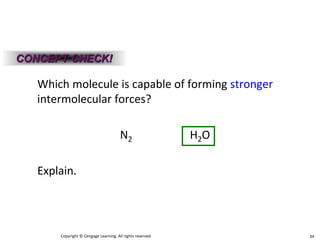
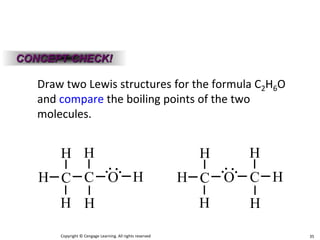
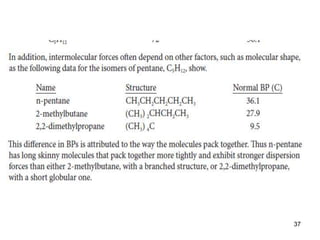
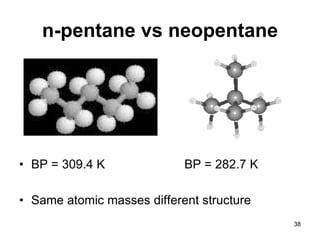
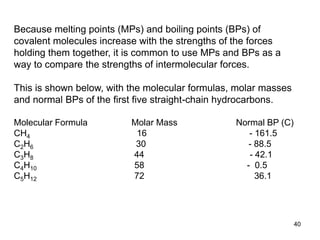
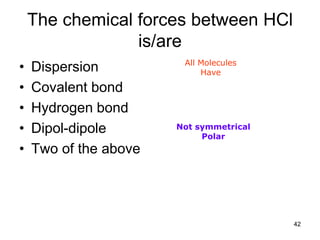
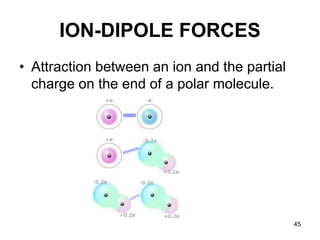
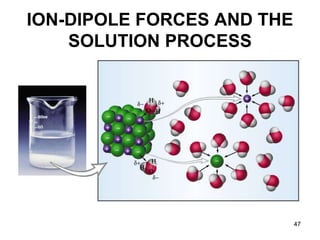
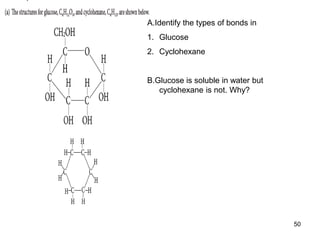
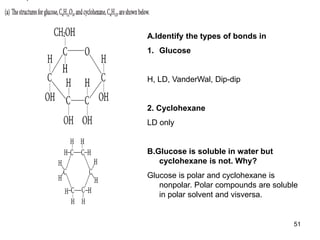
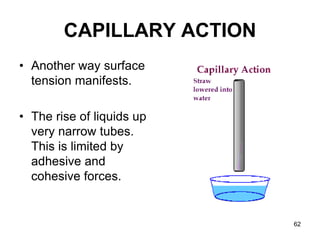
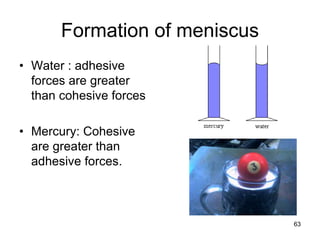
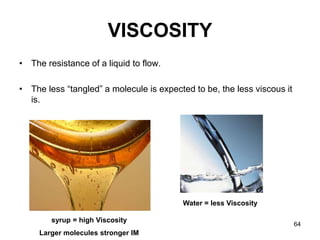
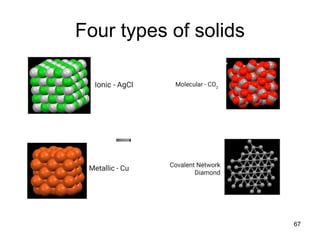
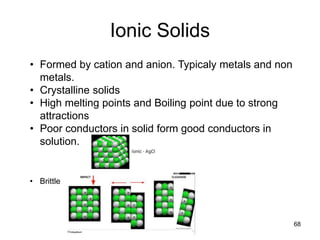
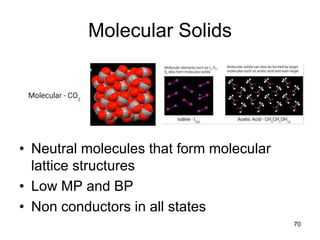
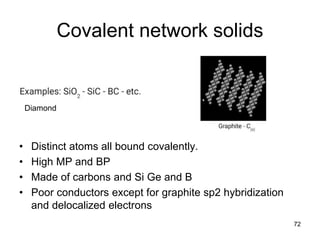
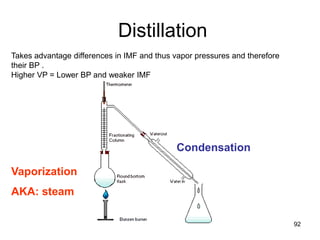
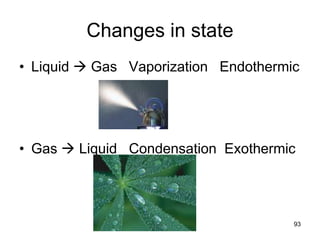
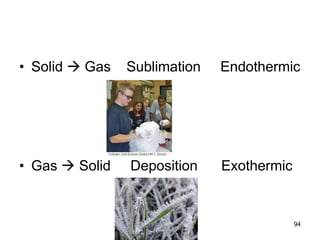
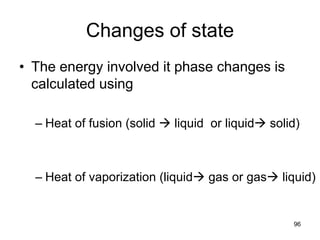
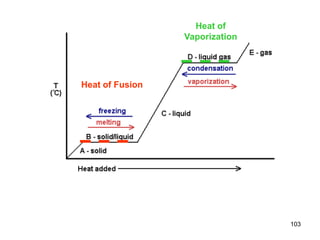
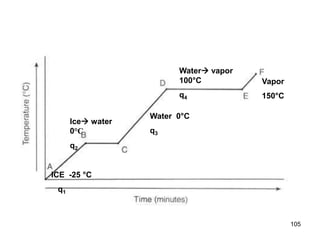
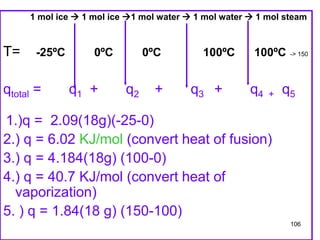
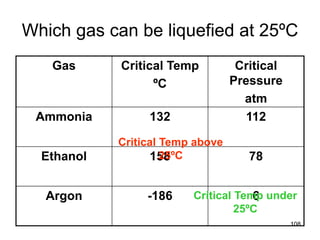
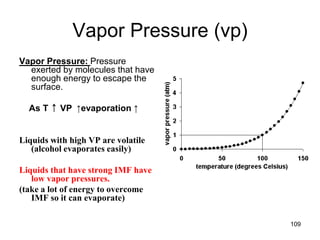
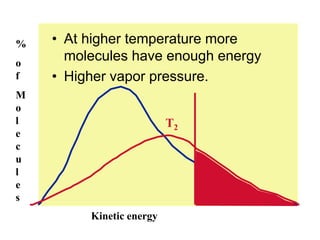
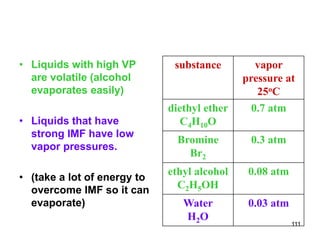
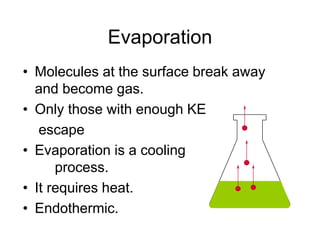
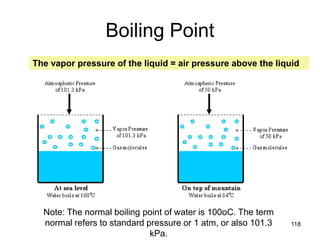
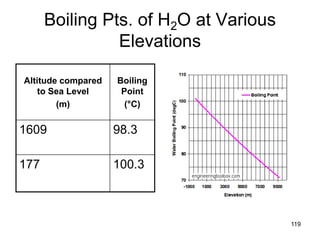
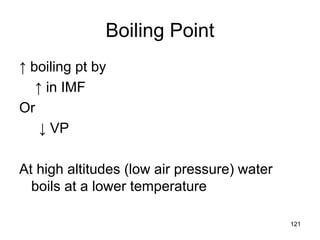
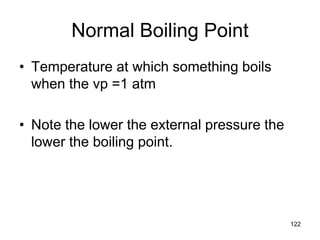
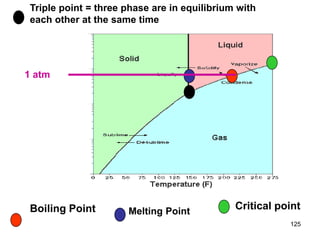
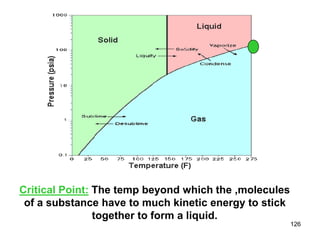
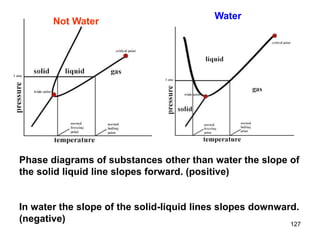
Okay, let's break this down step-by-step:
* We are given: 1 mole of ice at -25°C
* Heat of fusion of ice = 6.01 kJ/mol
* Heat of vaporization of water = 40.7 kJ/mol
* Specific heat of ice = 2.09 J/g°C
* Specific heat of water = 4.18 J/g°C
1) Heat ice from -25°C to 0°C:
Q = m * c * ΔT
Q = (18 g) * (2.09 J/g°C) * (25°C) = 903 J
2) Heat