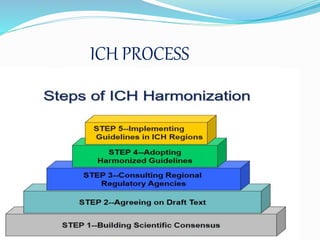
The International Conference on Harmonization (ICH) brings together regulatory authorities and pharmaceutical industries from Europe, Japan, and the United States to discuss scientific and technical aspects of drug registration. ICH aims to harmonize technical requirements for pharmaceutical registration to ensure safety, quality and efficacy while avoiding redundant testing. ICH has produced numerous guidelines on quality, safety, efficacy and multidisciplinary topics that are implemented by regulatory agencies in ICH regions and used globally to streamline drug development and approval processes.