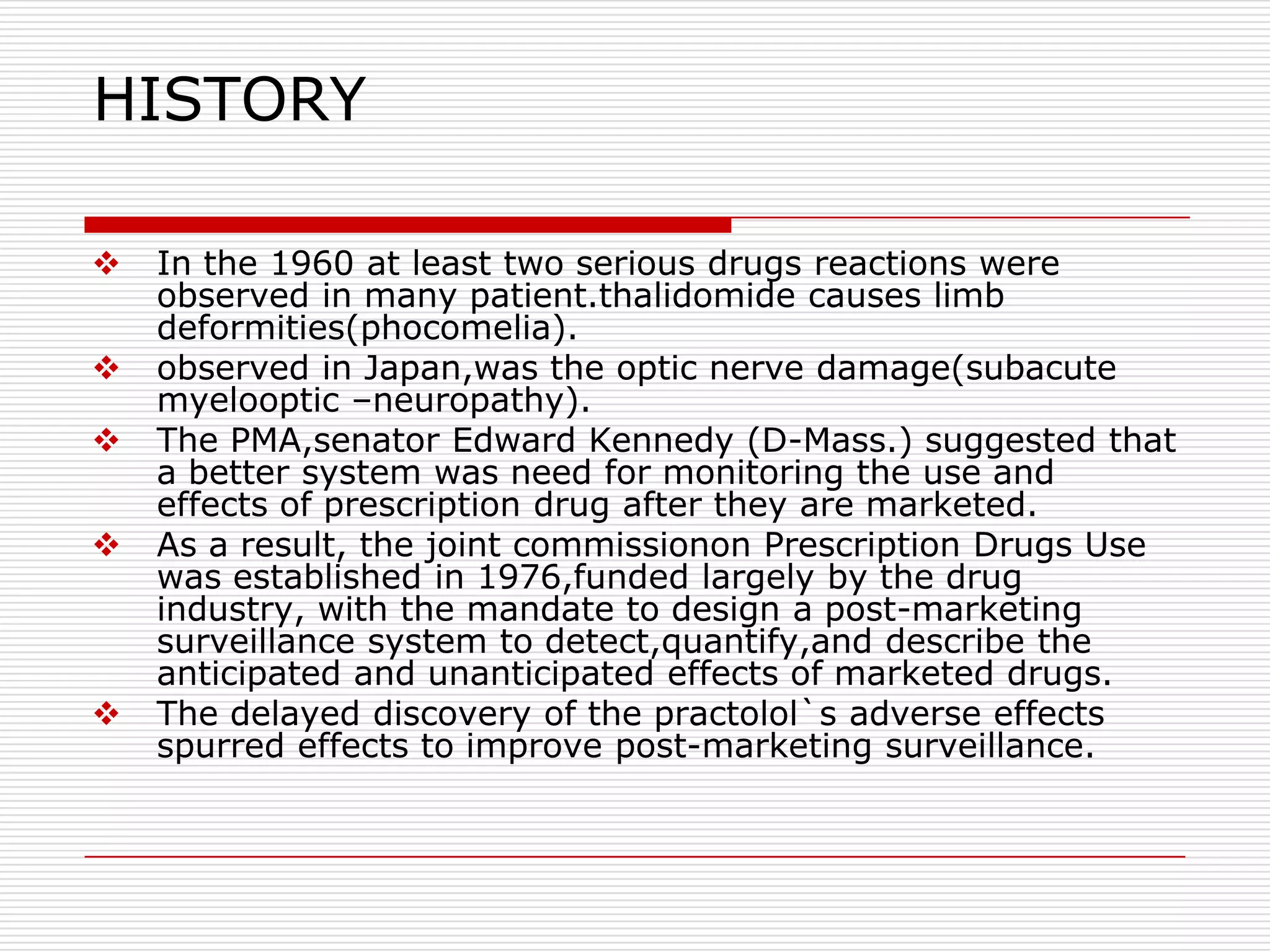
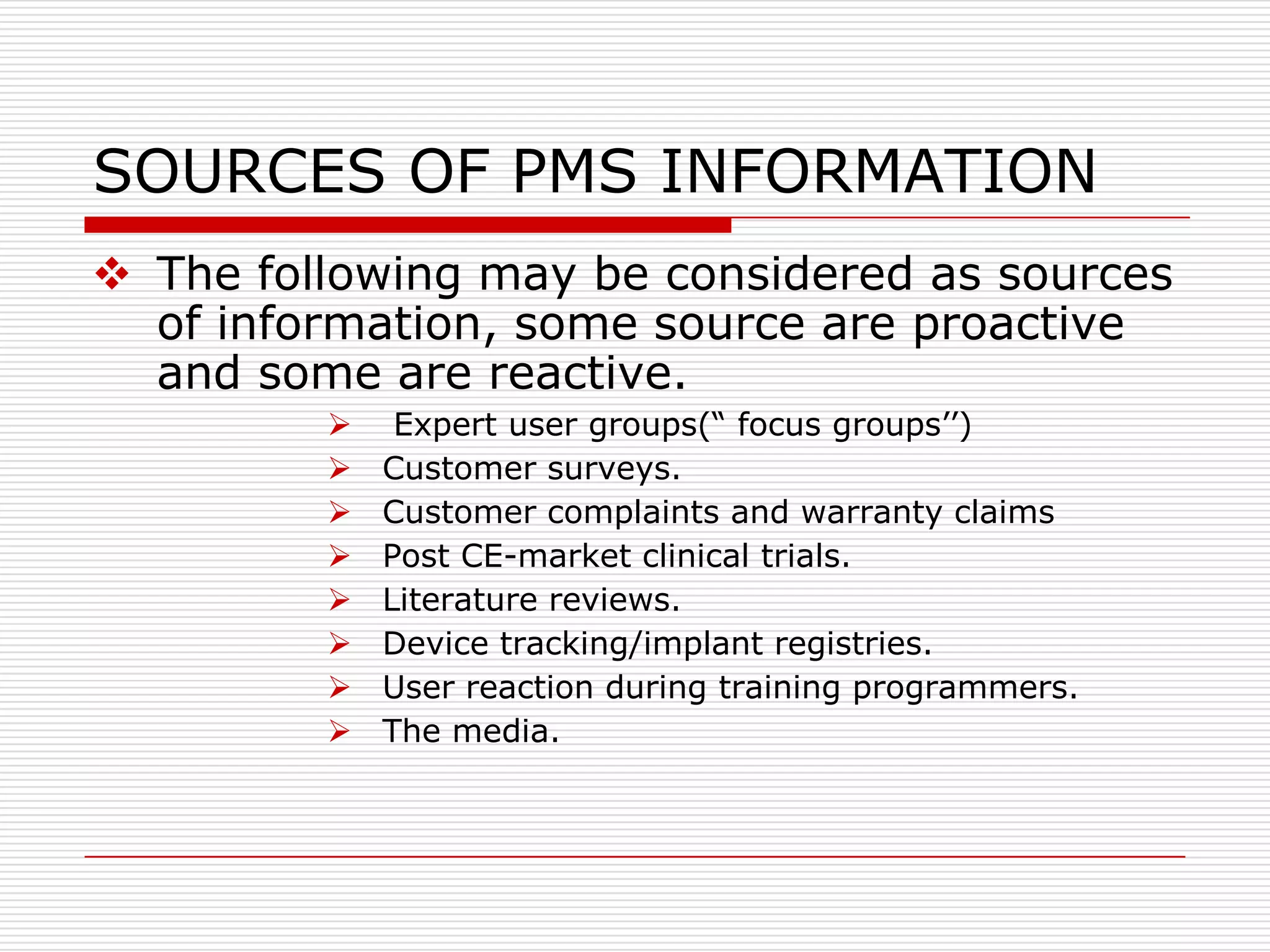
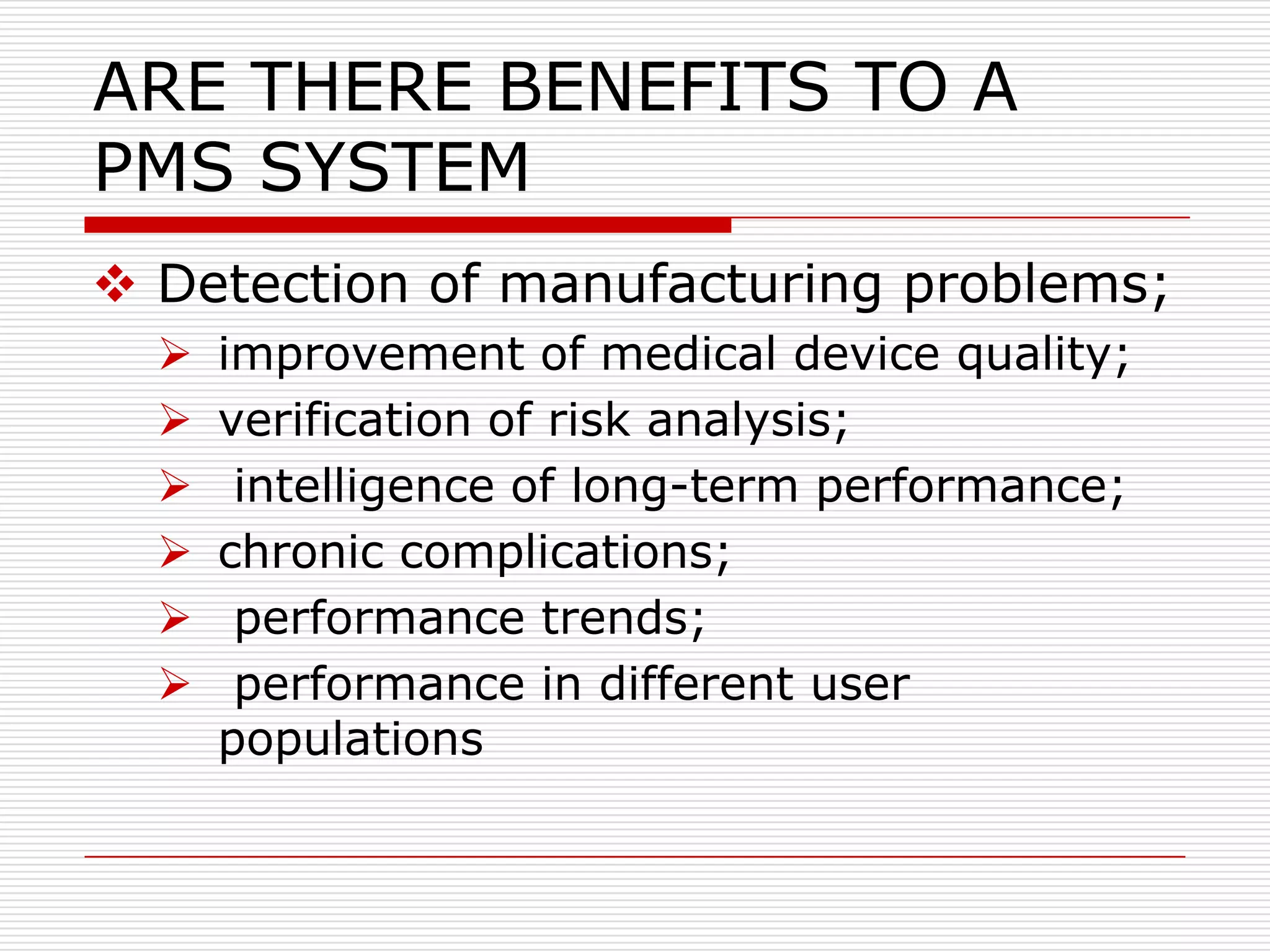
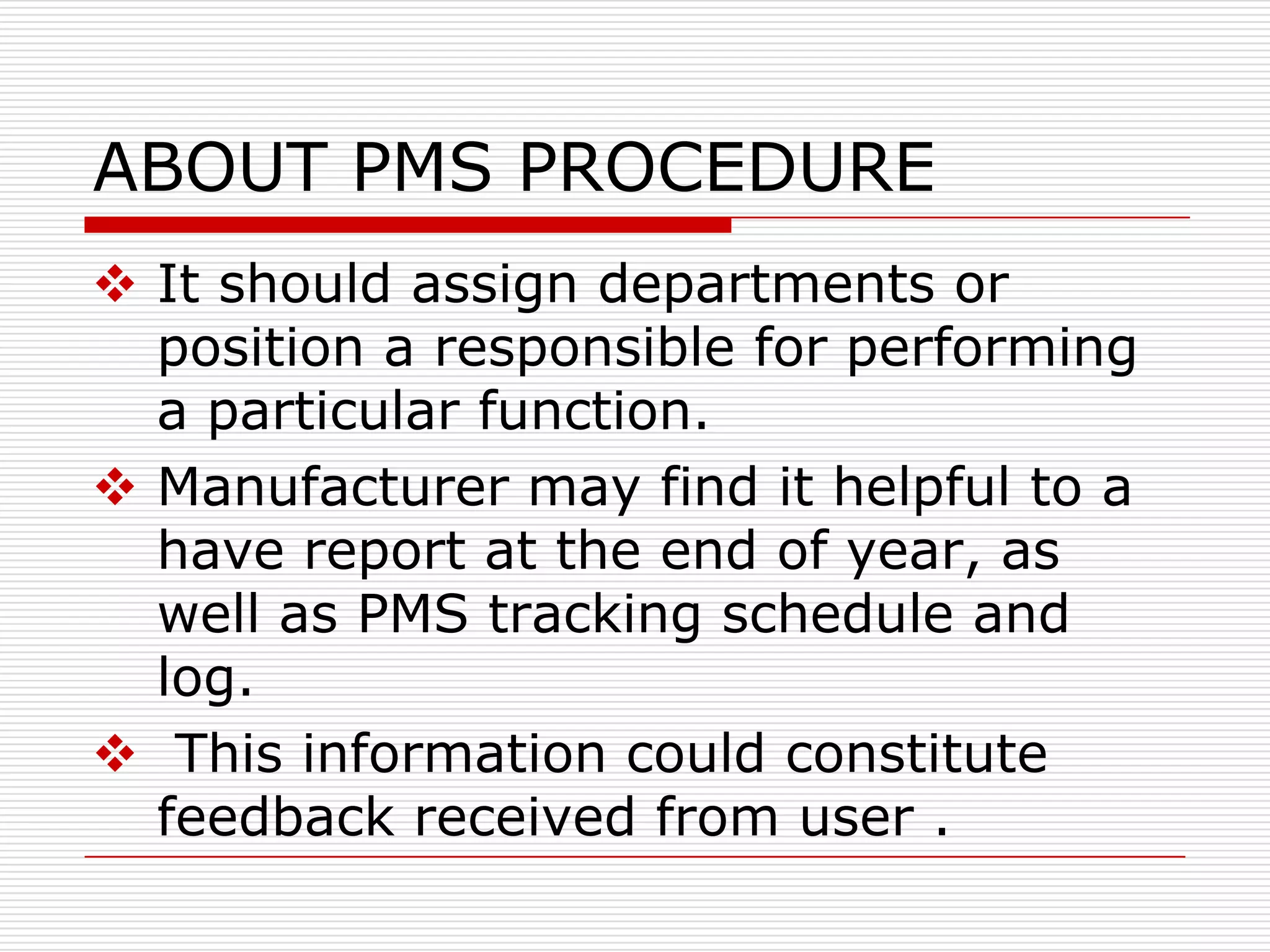
This document discusses post-marketing surveillance (PMS), which involves monitoring the safety of pharmaceutical drugs and medical devices after they have been approved and released on the market. PMS is important because pre-approval clinical trials involve limited numbers of patients and cannot detect all potential adverse effects. The document outlines the history of PMS, sources of PMS information, benefits of PMS systems, methods of surveillance including spontaneous reporting and cohort studies, and how manufacturers can establish PMS procedures and systems to gather feedback on their products.

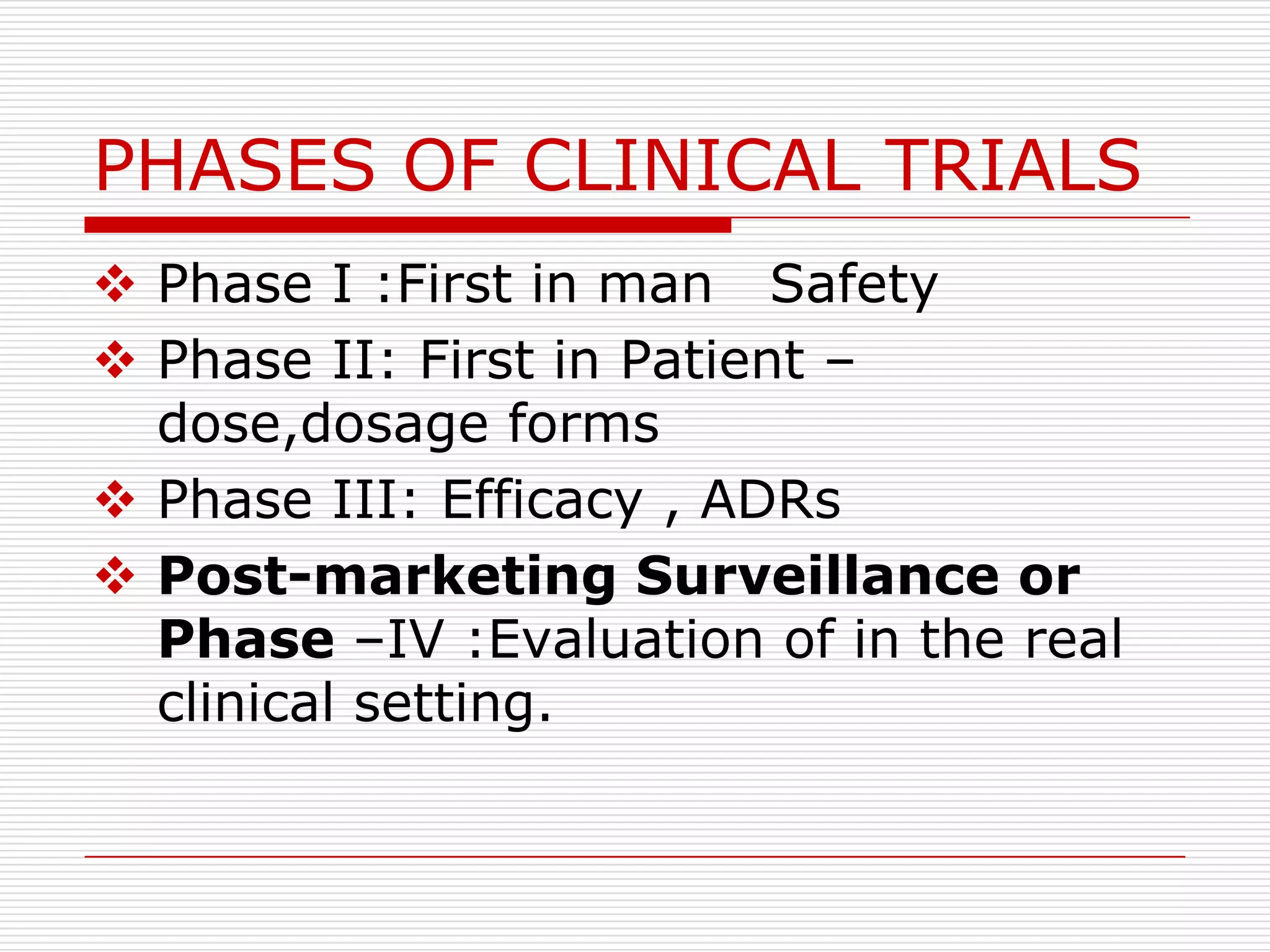

![POST-MARKETING SURVEILLANCE
No fixed duration/Patient population
Starts immediately after marketing
Report all ADRs
Help to detect
Rare ADRs
Drug interaction
Also new uses for drugs[sometimes called
Phase V]](https://image.slidesharecdn.com/pmsnew-120921014837-phpapp02/75/Post-marketing-servillence-5-2048.jpg)