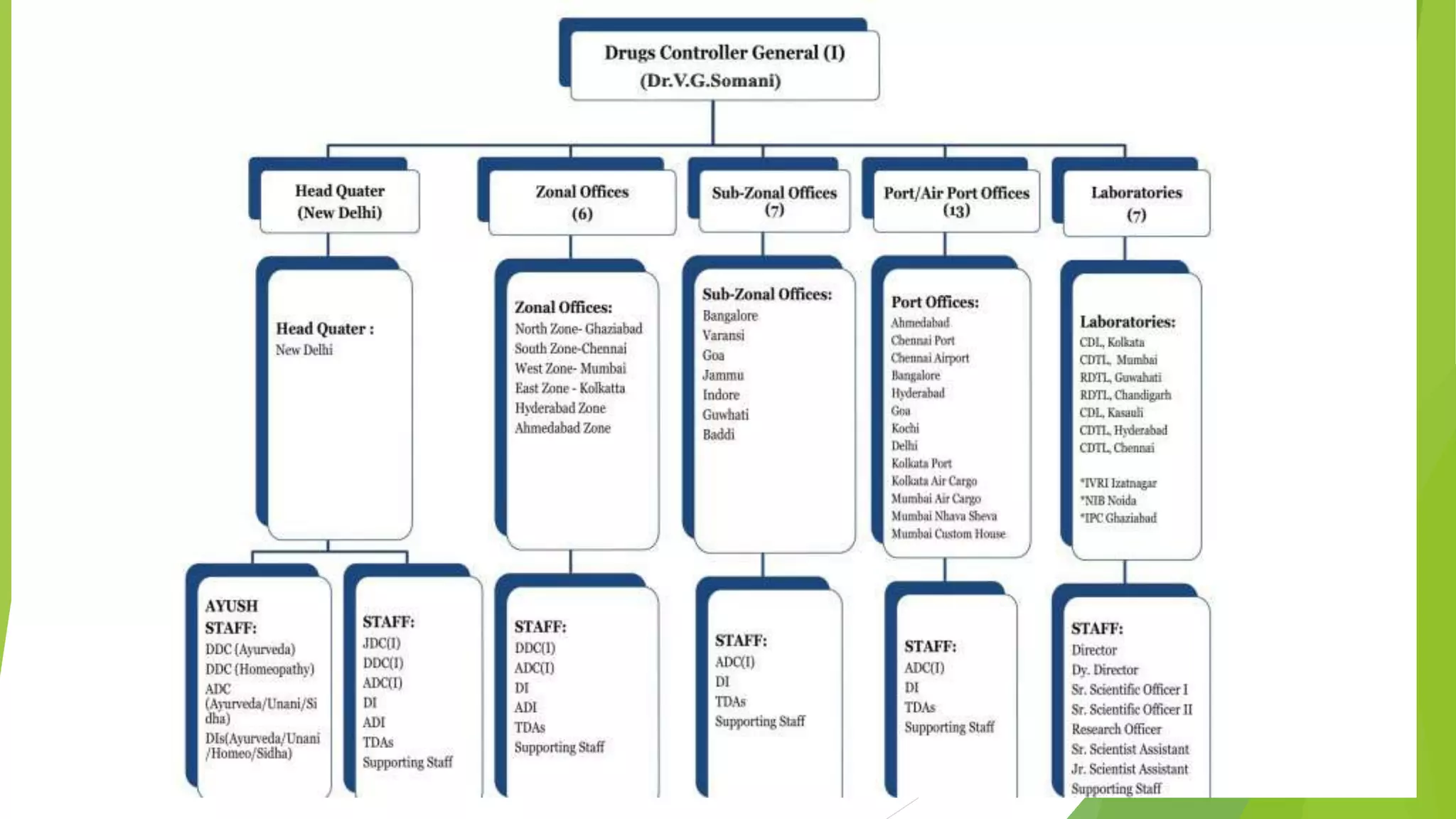
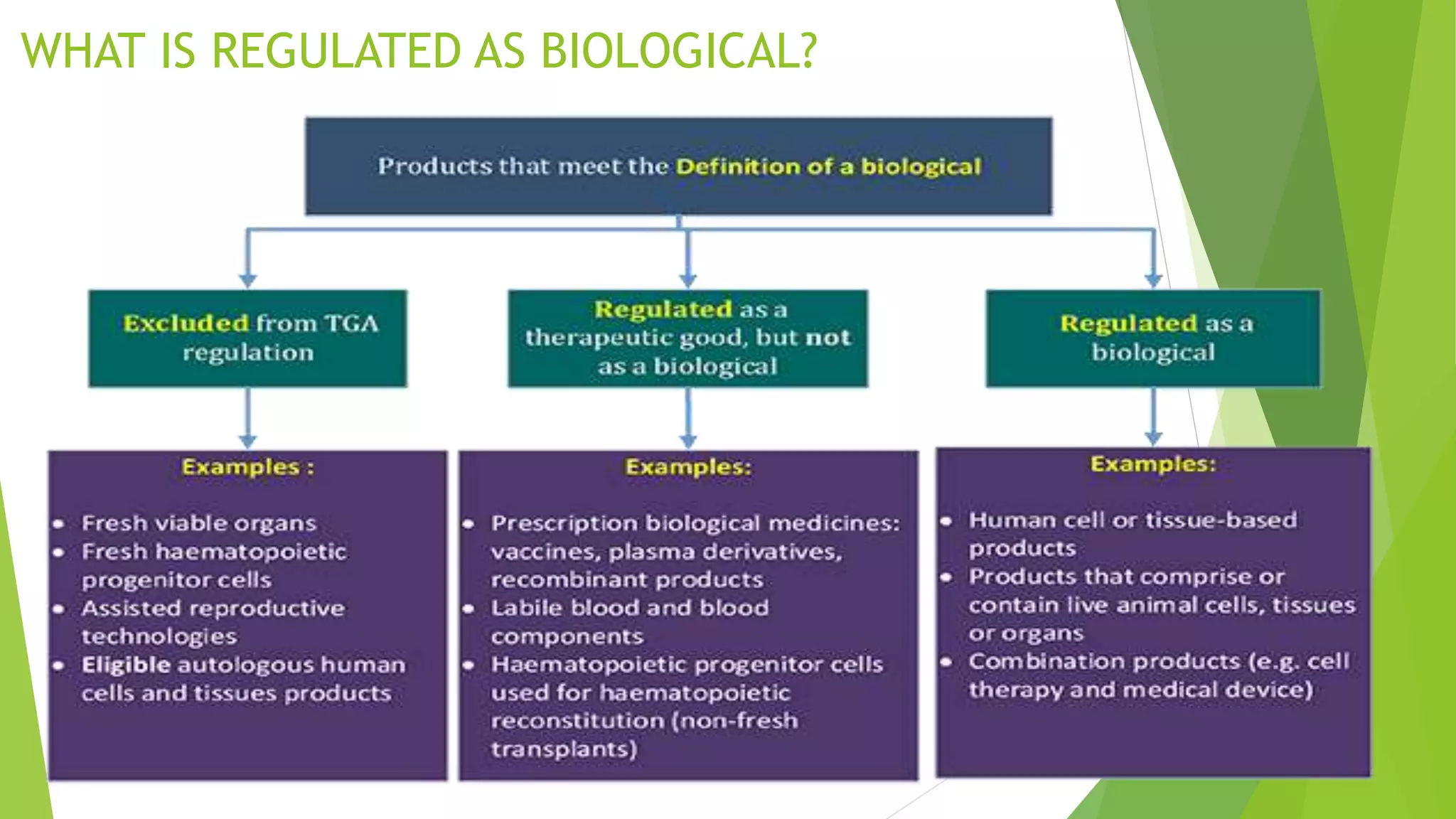
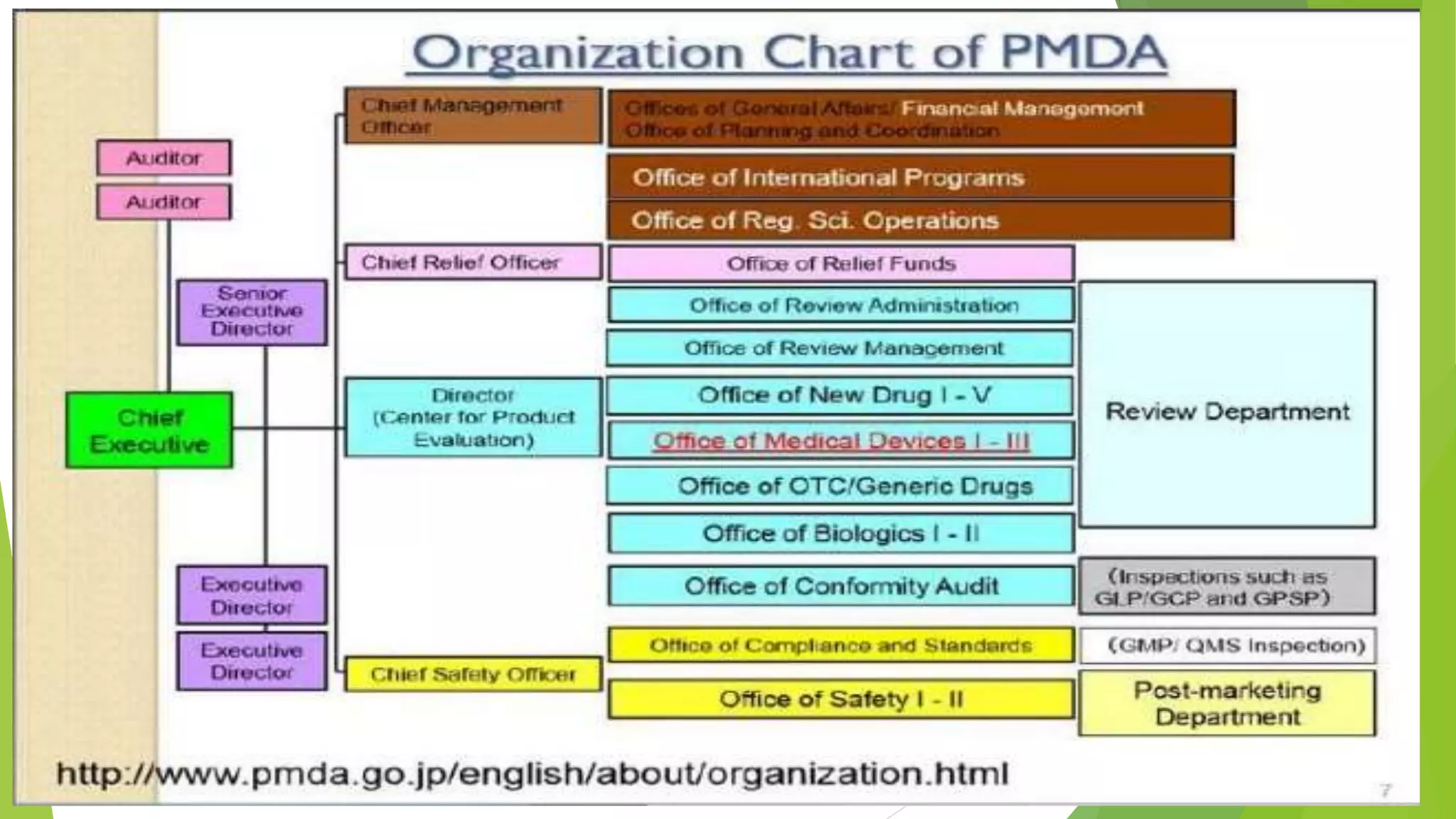
The document summarizes several regulatory agencies that regulate pharmaceutical products around the world. It discusses the roles and functions of the CDSCO in India, FDA in the United States, TGA in Australia, Health Canada, and MHRA in the UK. For each agency, it provides information on their goals, activities related to drug approval and regulation, and key application forms.