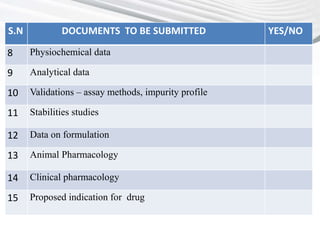
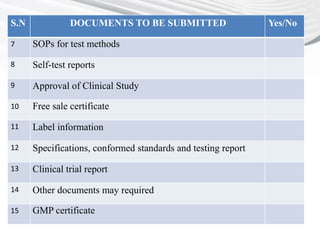
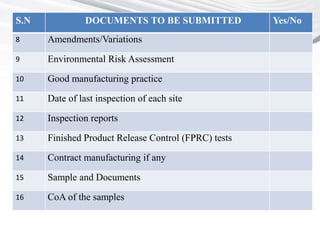
This document provides a checklist of documents required for obtaining market authorization in various BRICS countries. It introduces BRICS as an emerging market comprising Brazil, Russia, India, China and South Africa. Reasons for the emergence of BRICS markets include declining growth in developed markets and availability of patient populations in emerging markets. The checklist then outlines the documents required for market authorization from the regulatory authorities in India, China, South Africa and other BRICS countries. These include application forms, composition details, clinical trial reports, GMP certificates, and other product-specific documents.