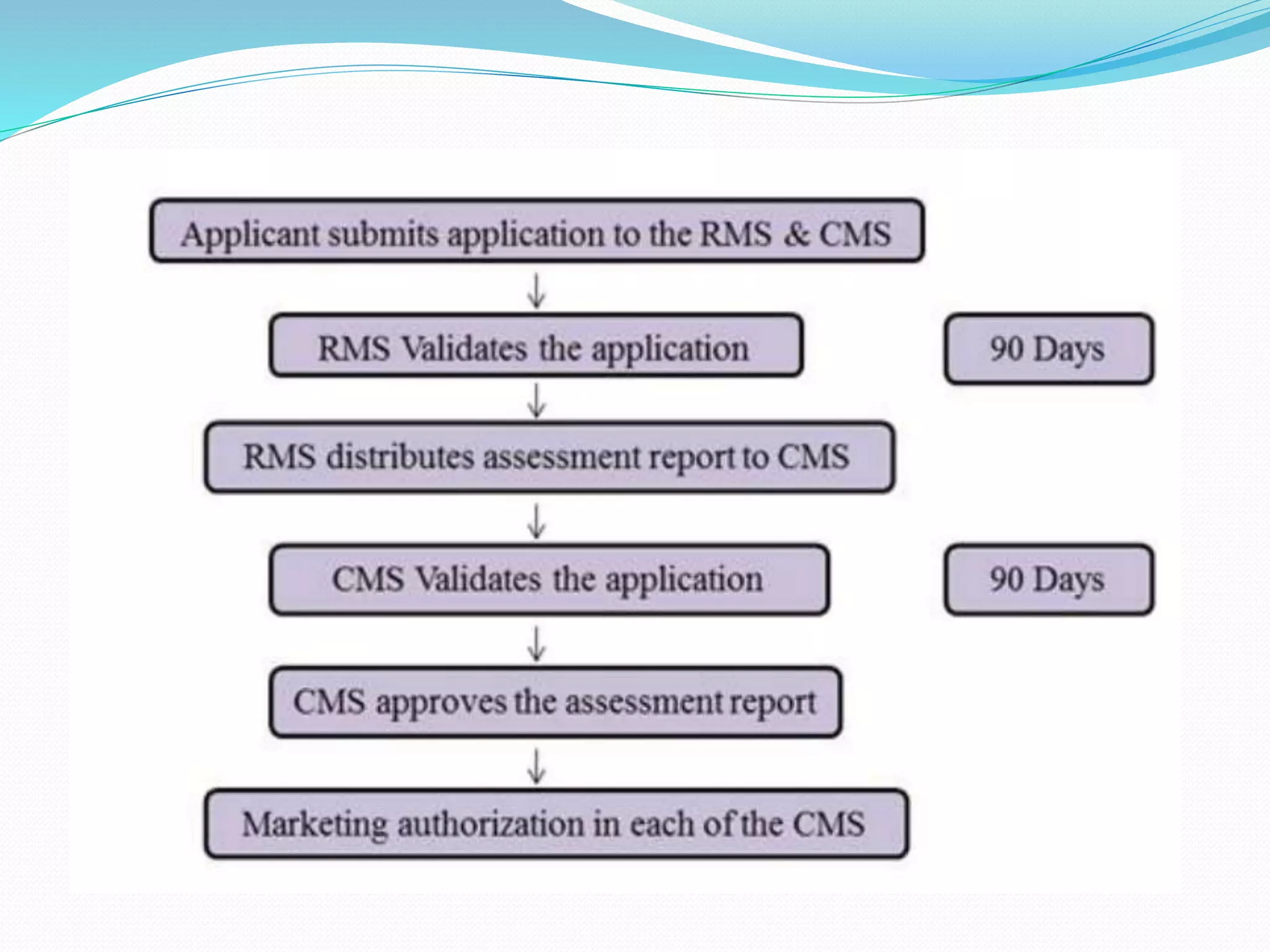
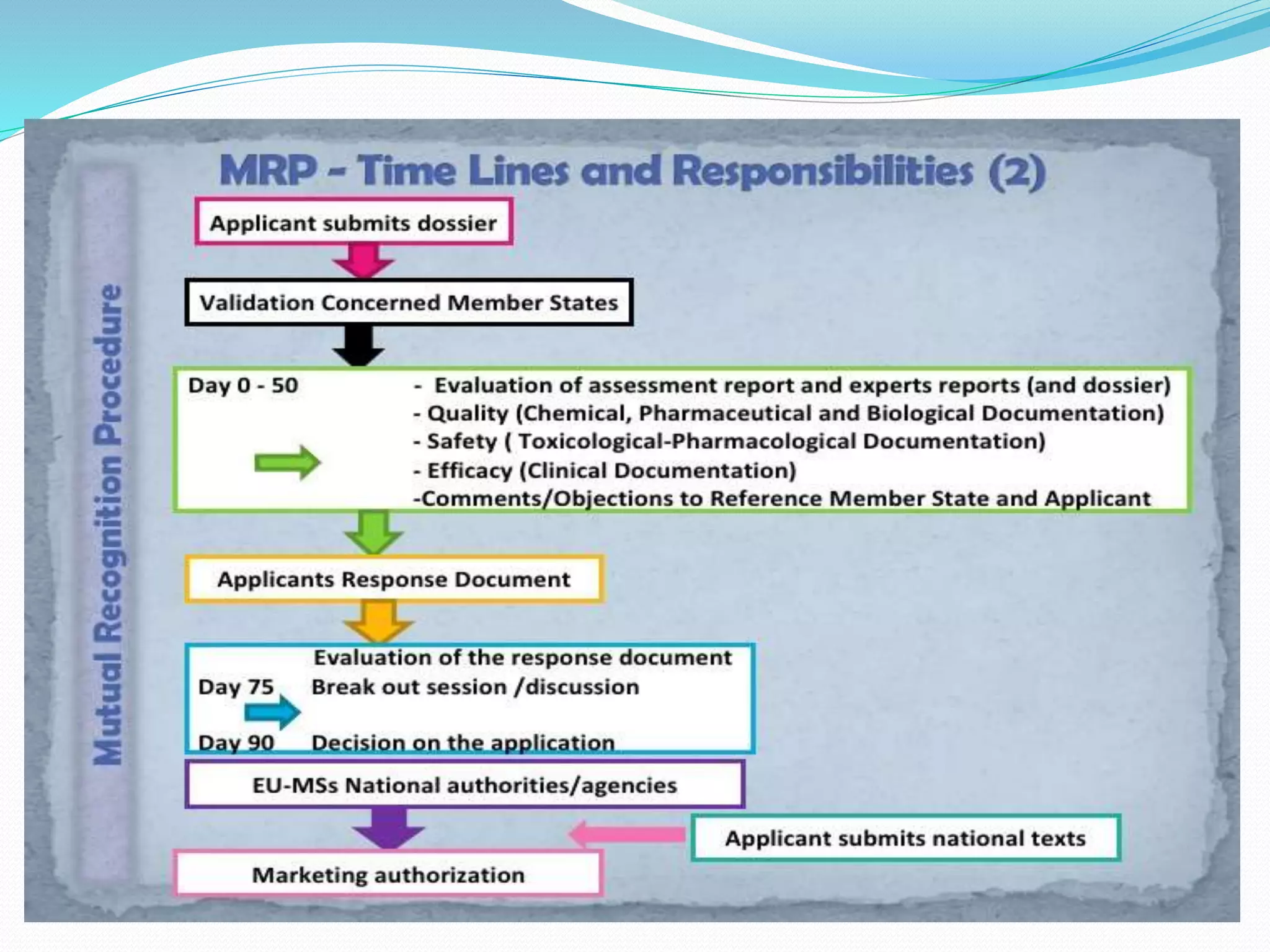
Mutual recognition is an EU principle that requires member states to allow goods legally sold in another member state to be sold in their own territory. This eliminates the need for duplicate testing and certification in importing countries. Mutual recognition agreements between countries or regions formalize this principle for international trade by designating conformity assessment bodies whose test results and certifications are mutually recognized. The EU-US MRA allows regulators to rely on each other's drug inspections to avoid duplication and increase efficiencies.