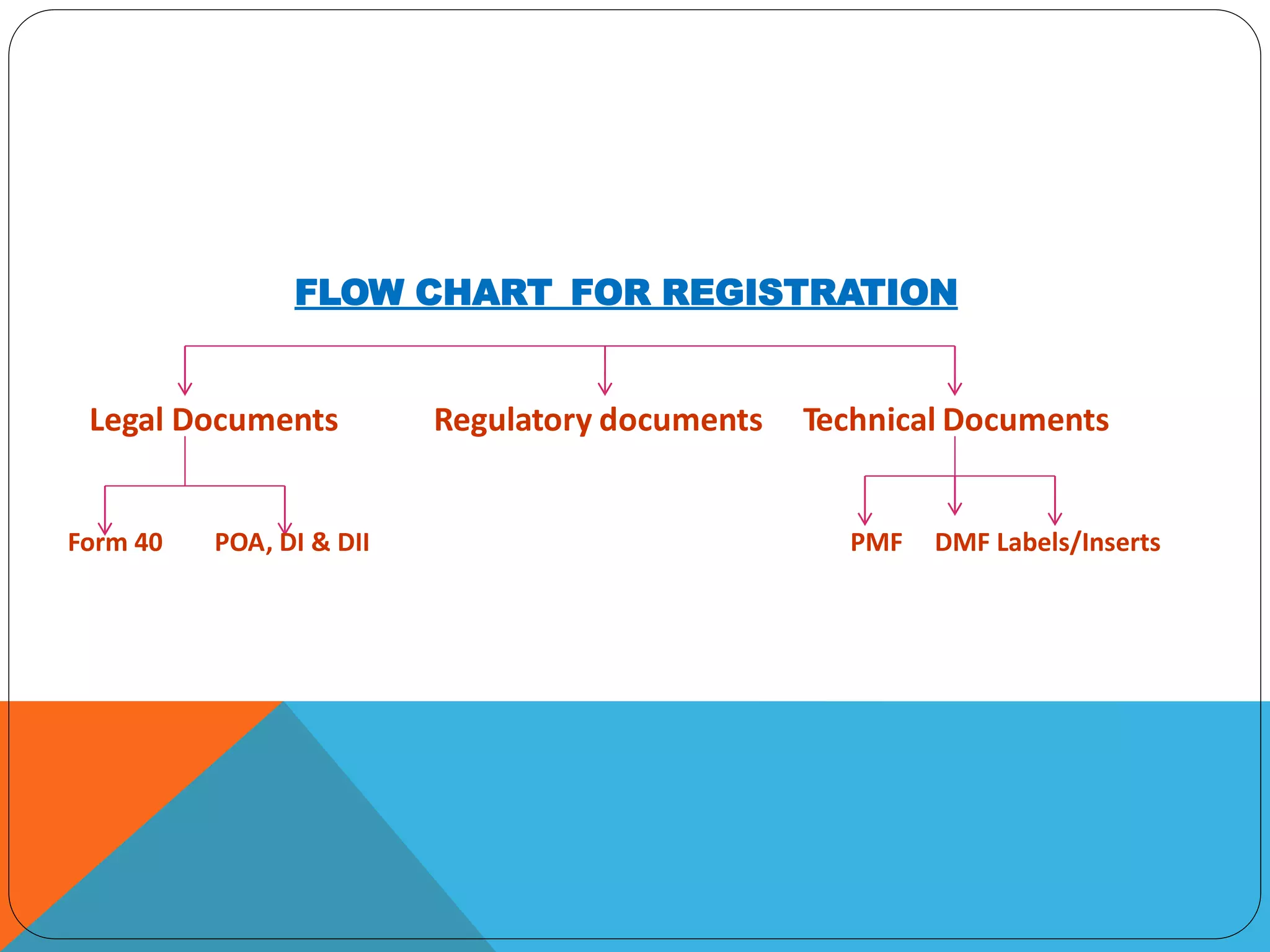
The document discusses India's drug regulatory system. The Drug Controller General of India regulates drugs and medical devices in the country to ensure quality, safety and efficacy. New drugs require approval through a New Drug Application process which involves submitting documentation on manufacturing, non-clinical studies, and clinical trials for review. It takes about a year to review an NDA and various forms and fees are involved in the approval and import license application processes.
![New Drug Approval
[NDA]](https://image.slidesharecdn.com/newdrugapproval-180519043220/75/New-drug-approval-1-2048.jpg)



![FLOW C
Biologica
Co
• Veterinary Pr
HART FOR New Drug Approval
(NDA)
ls Vaccines New drugs/Devices
mmon Technical documents
(Modules 1-5)
[CTD]
oduct - CTD Modules 1-4
- Noc from Animal Husbandry](https://image.slidesharecdn.com/newdrugapproval-180519043220/75/New-drug-approval-5-2048.jpg)