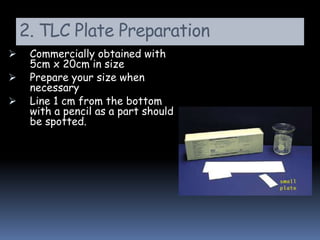
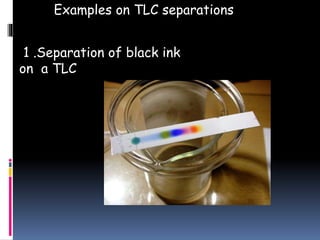
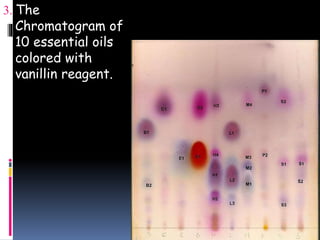
This document provides an introduction to thin layer chromatography (TLC). TLC is a technique used to separate non-volatile mixtures based on differences in compound affinity for the stationary and mobile phases. The document describes the basic components and process of TLC, including that silica plates are commonly used as the stationary phase, and volatile organic solvents as the mobile phase. Samples are spotted onto the plate and developed in a solvent, then visualized under UV light or with chemical reagents to identify separated compounds.