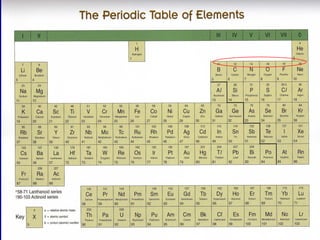
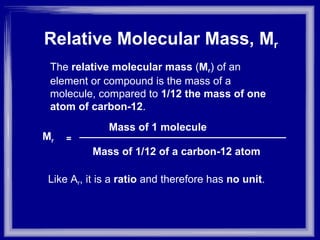
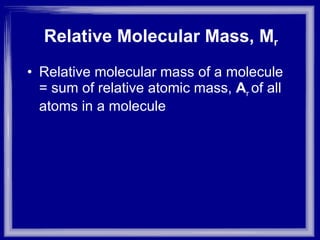
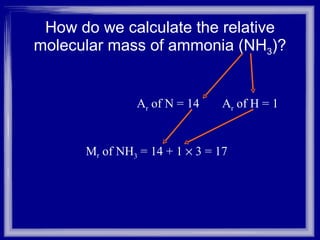
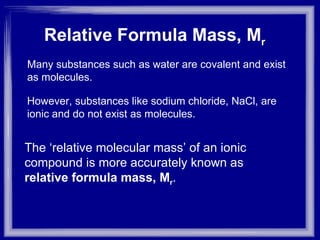
The document discusses relative atomic mass (Ar) and relative molecular/formula mass (Mr). It explains that Ar is a ratio of the mass of a given atom to 1/12 the mass of one carbon-12 atom, with no units. Mr is similarly defined for molecules and ionic compounds. The document provides examples of calculating Ar and Mr values for various elements and compounds from their atomic masses.