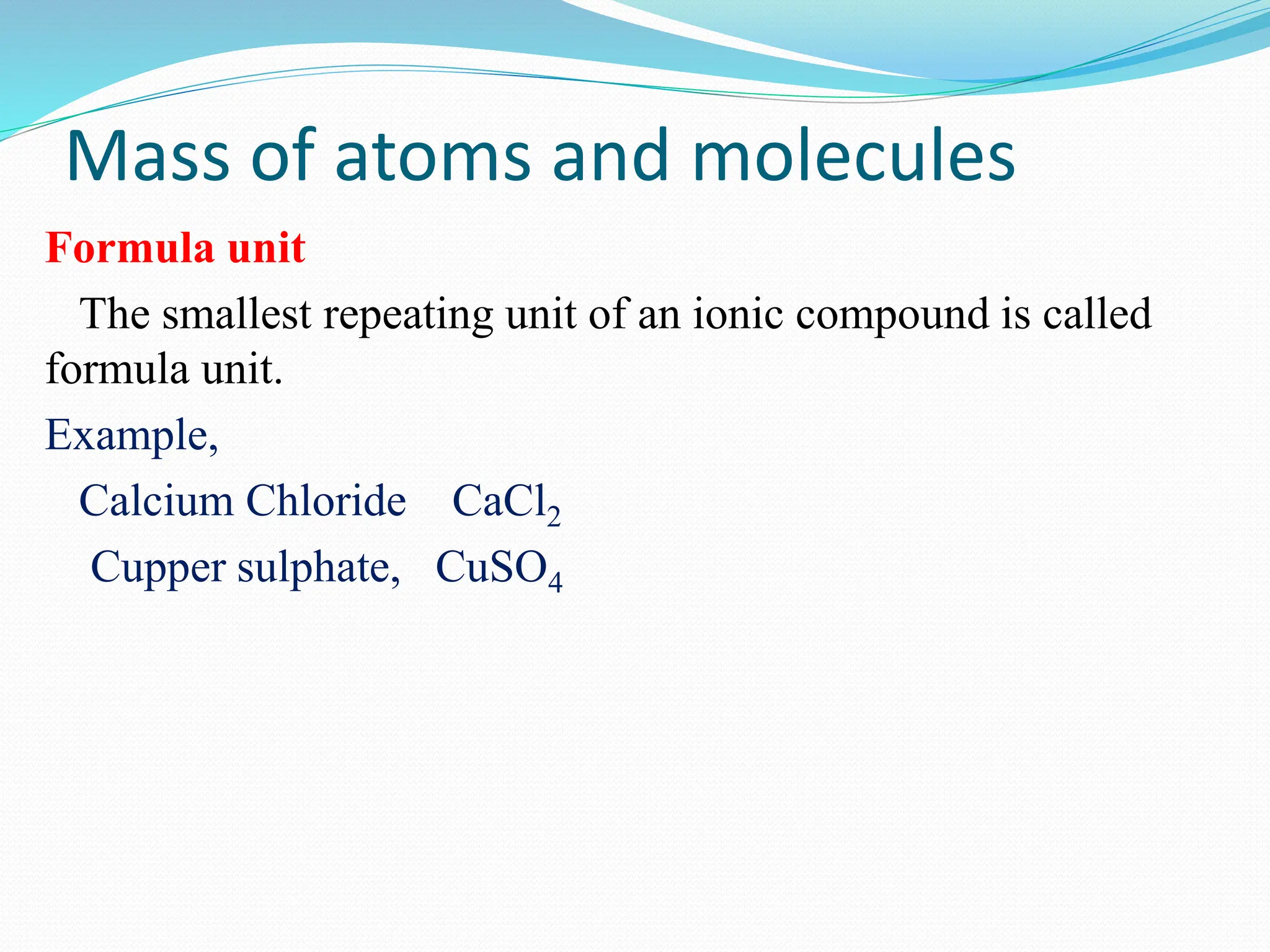
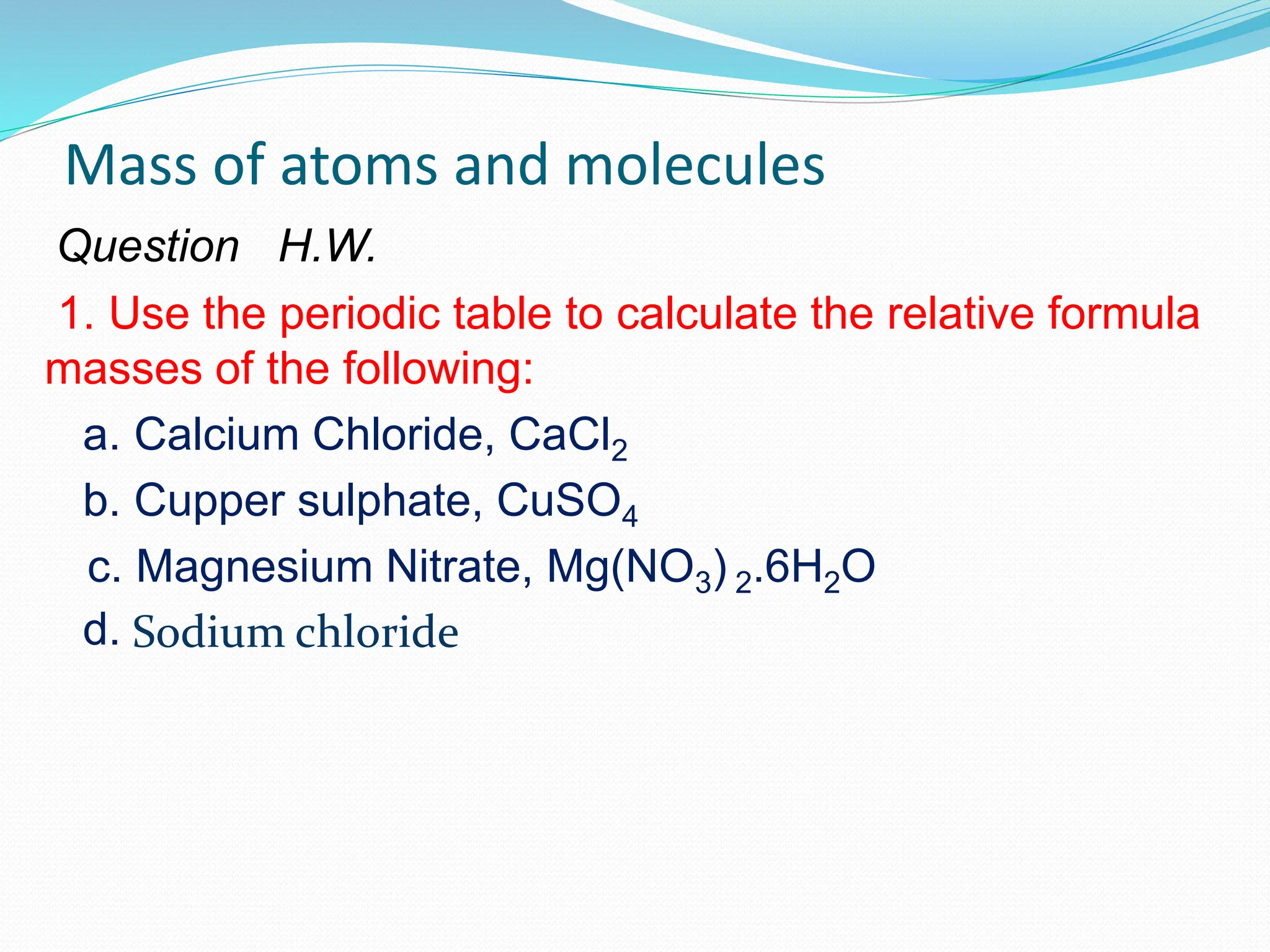
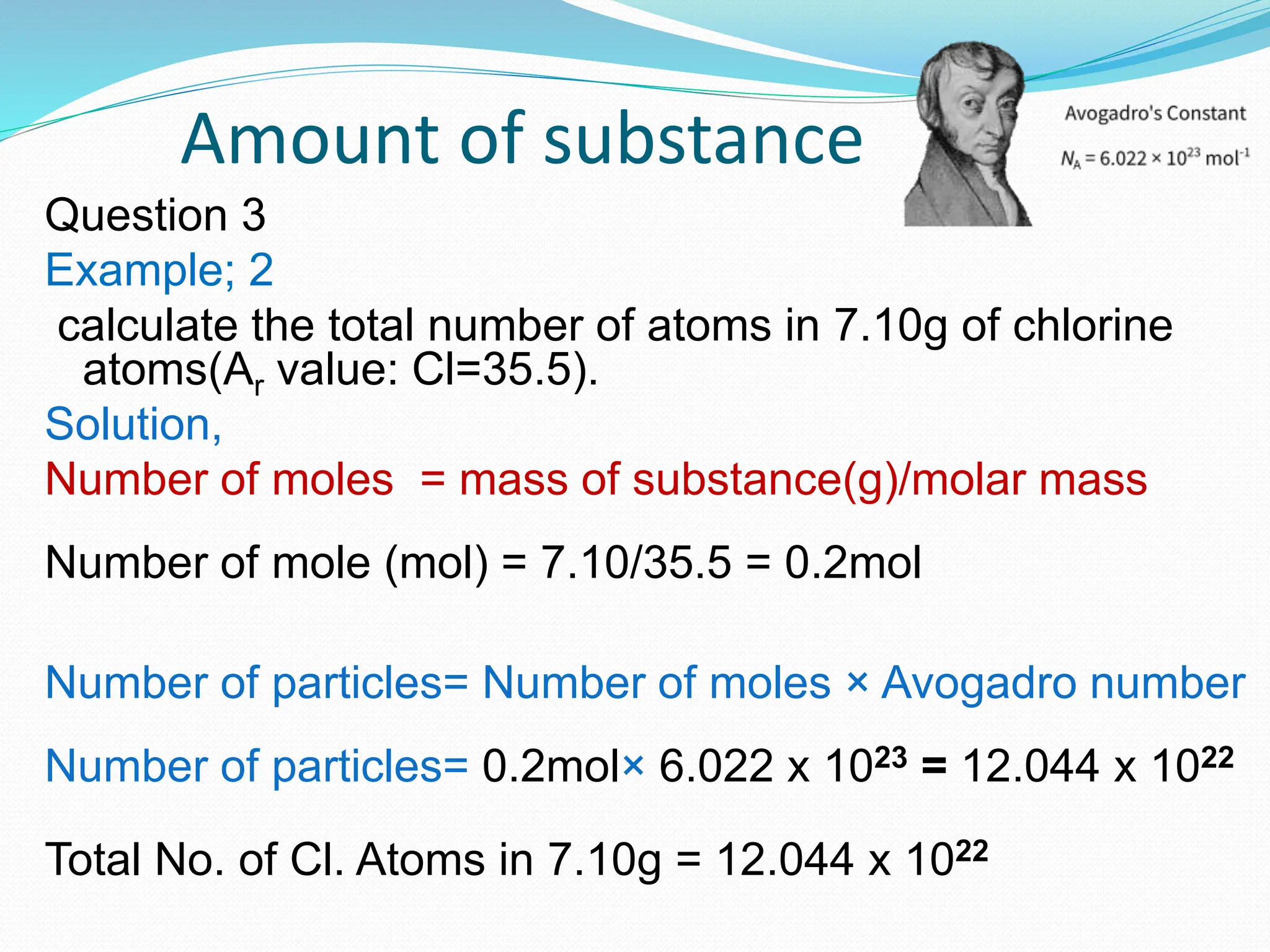
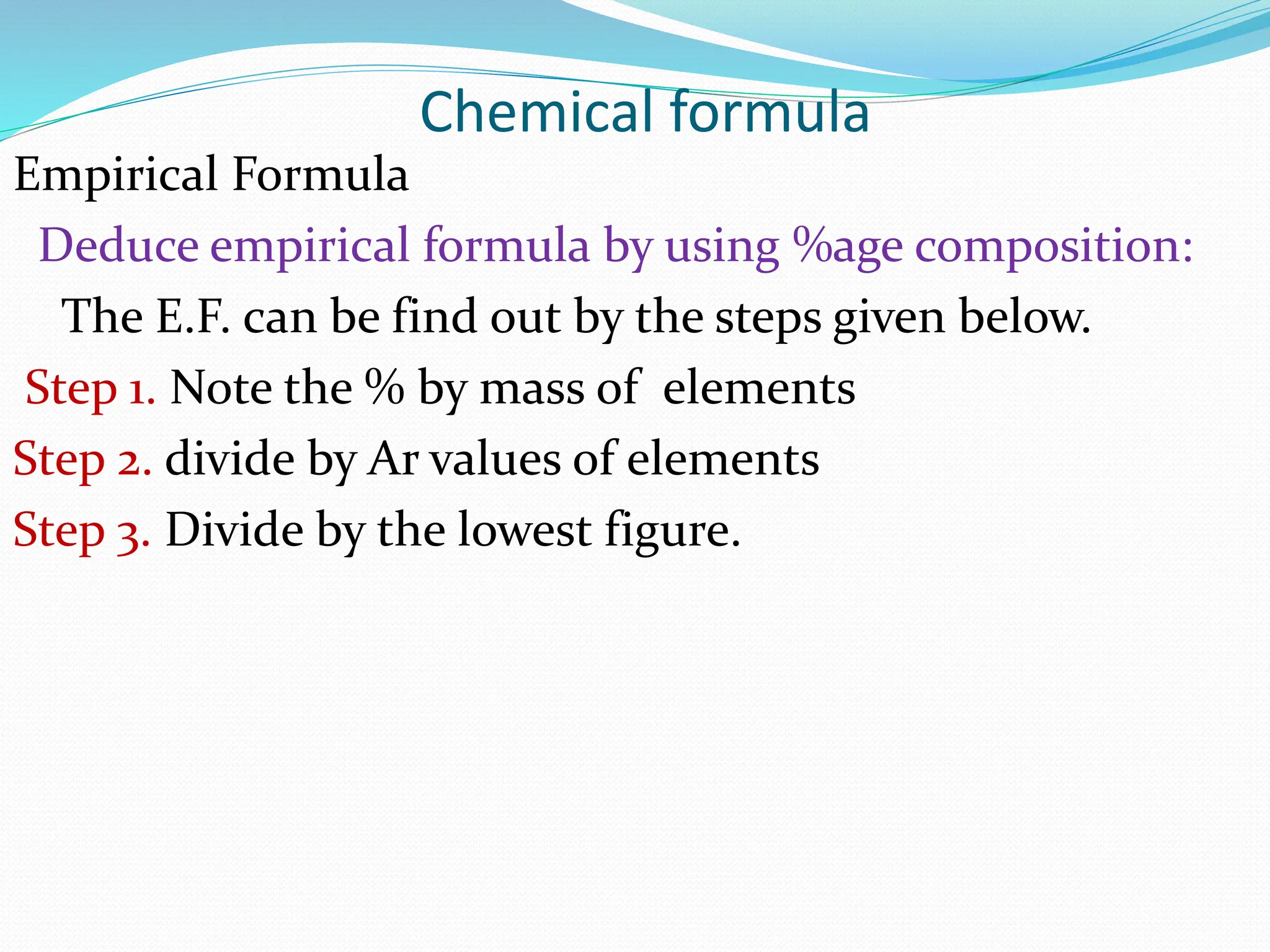
This document provides an introduction and table of contents for a chemistry course book on Cambridge International AS and A Level Chemistry. It covers topics like the mass of atoms and molecules, relative atomic masses, isotopic masses, amount of substance, mole calculation, chemical formulae, solutions, gas volume calculations, and more. The document gives definitions and examples for these concepts. It also provides sample problems and homework questions related to chemical calculations involving moles, masses, and chemical equations.