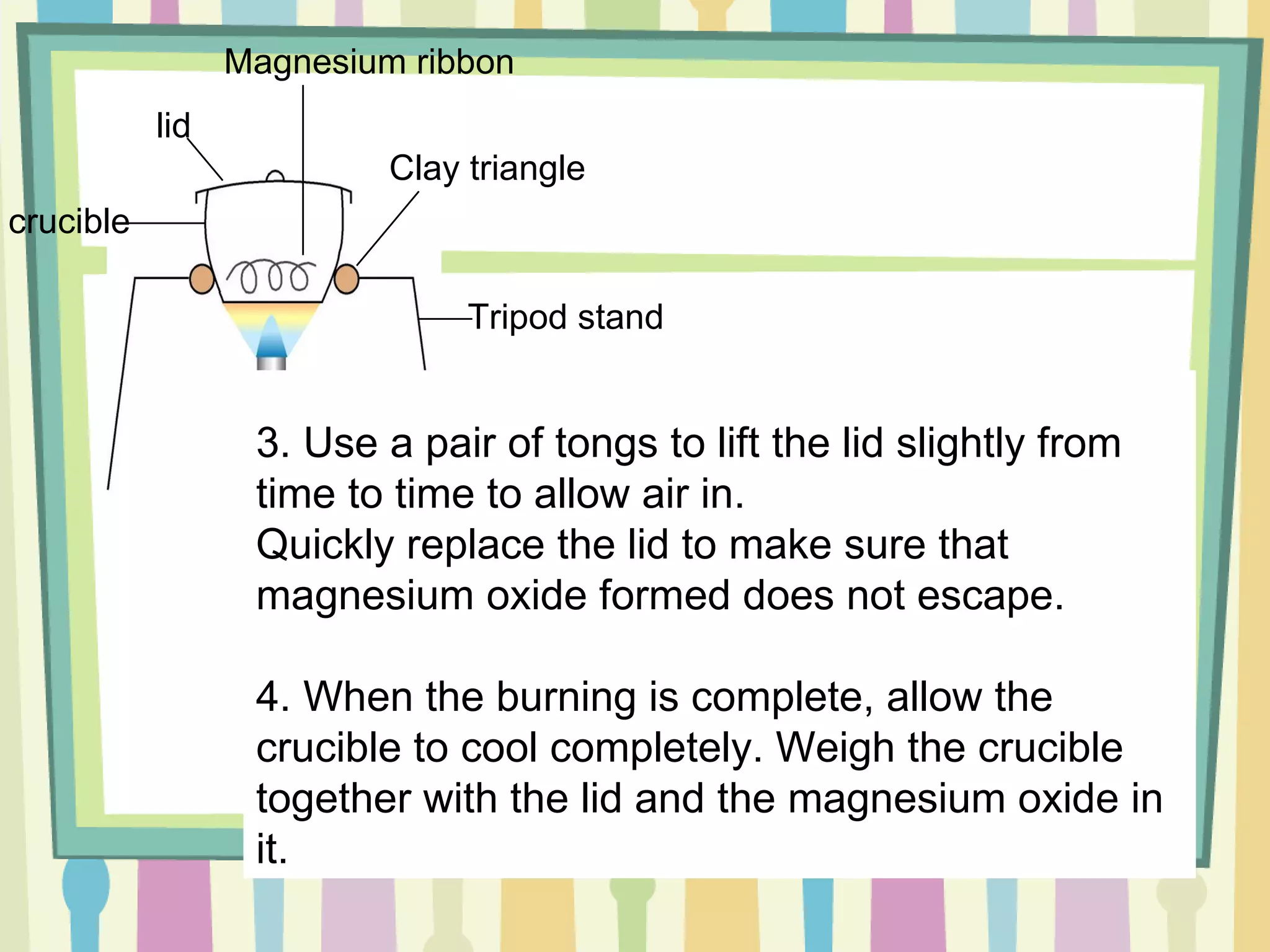
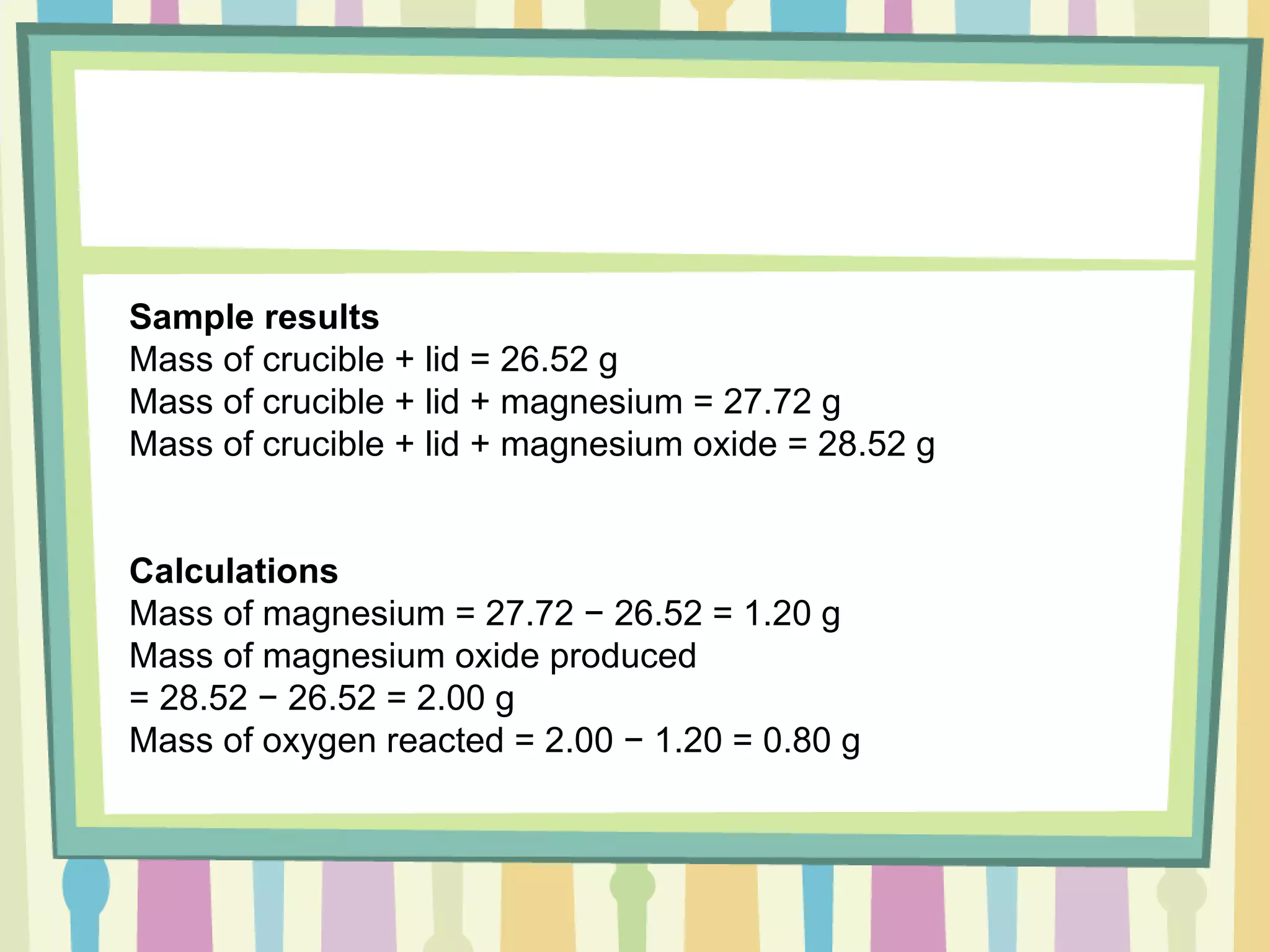
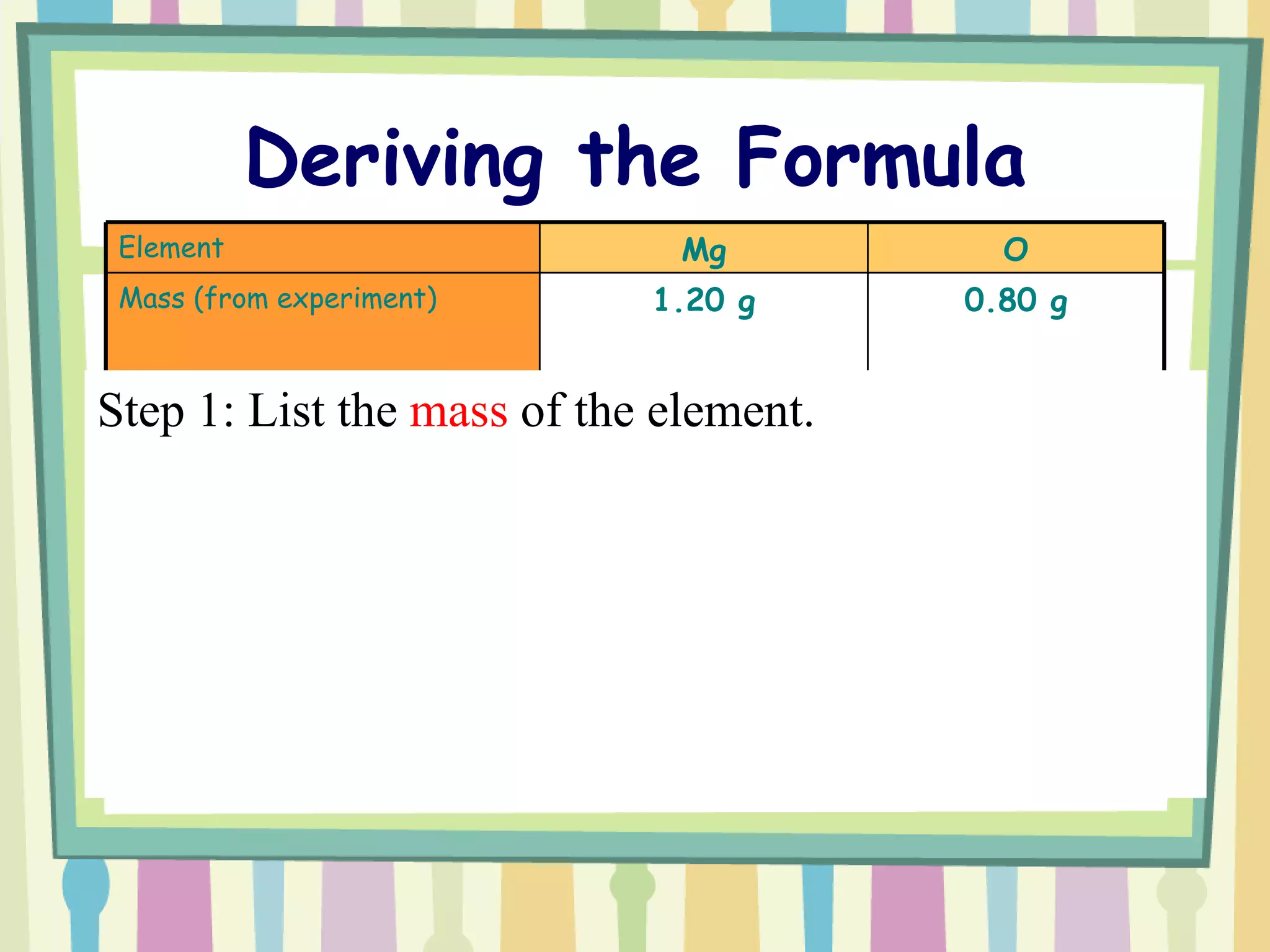
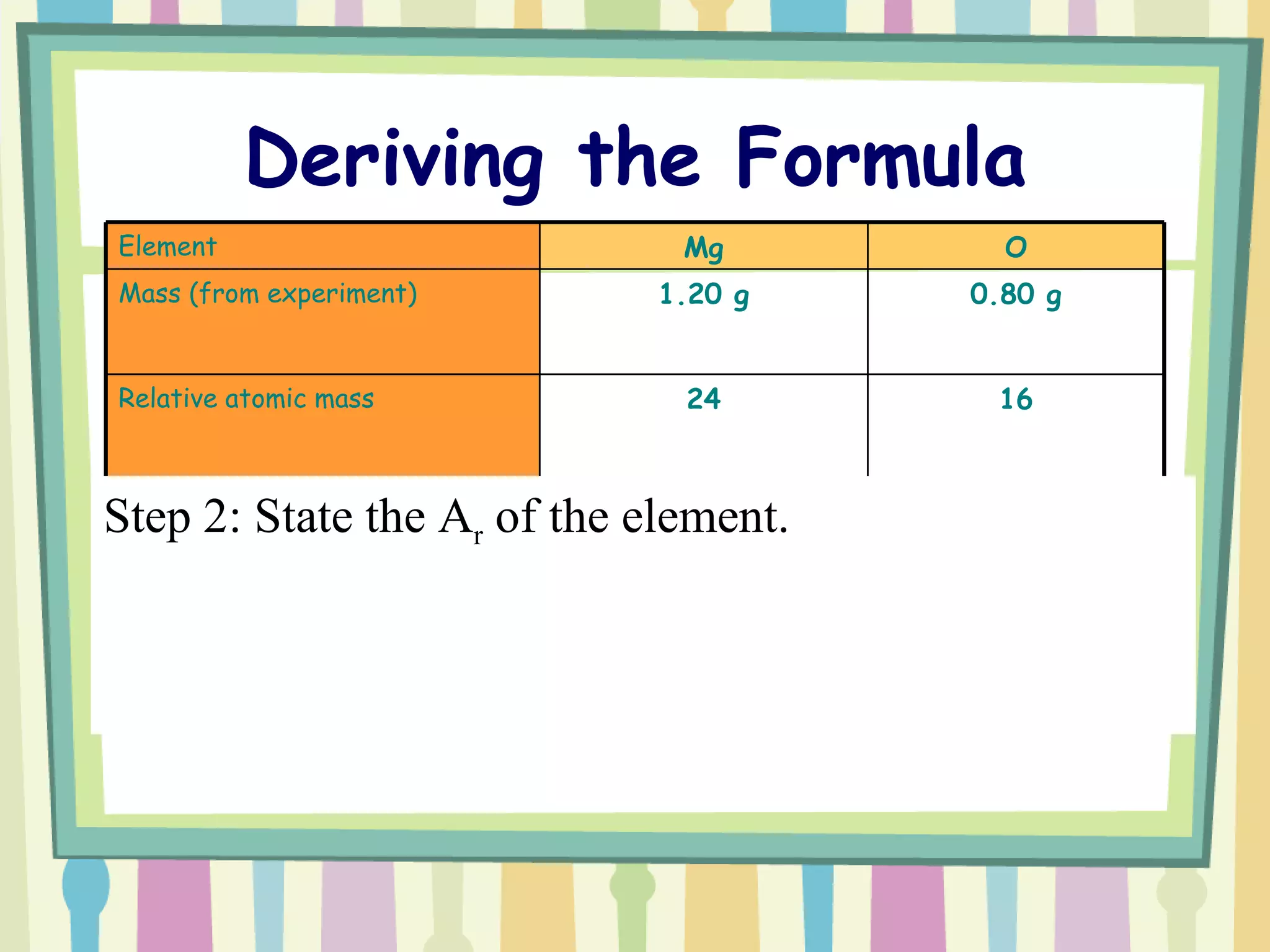
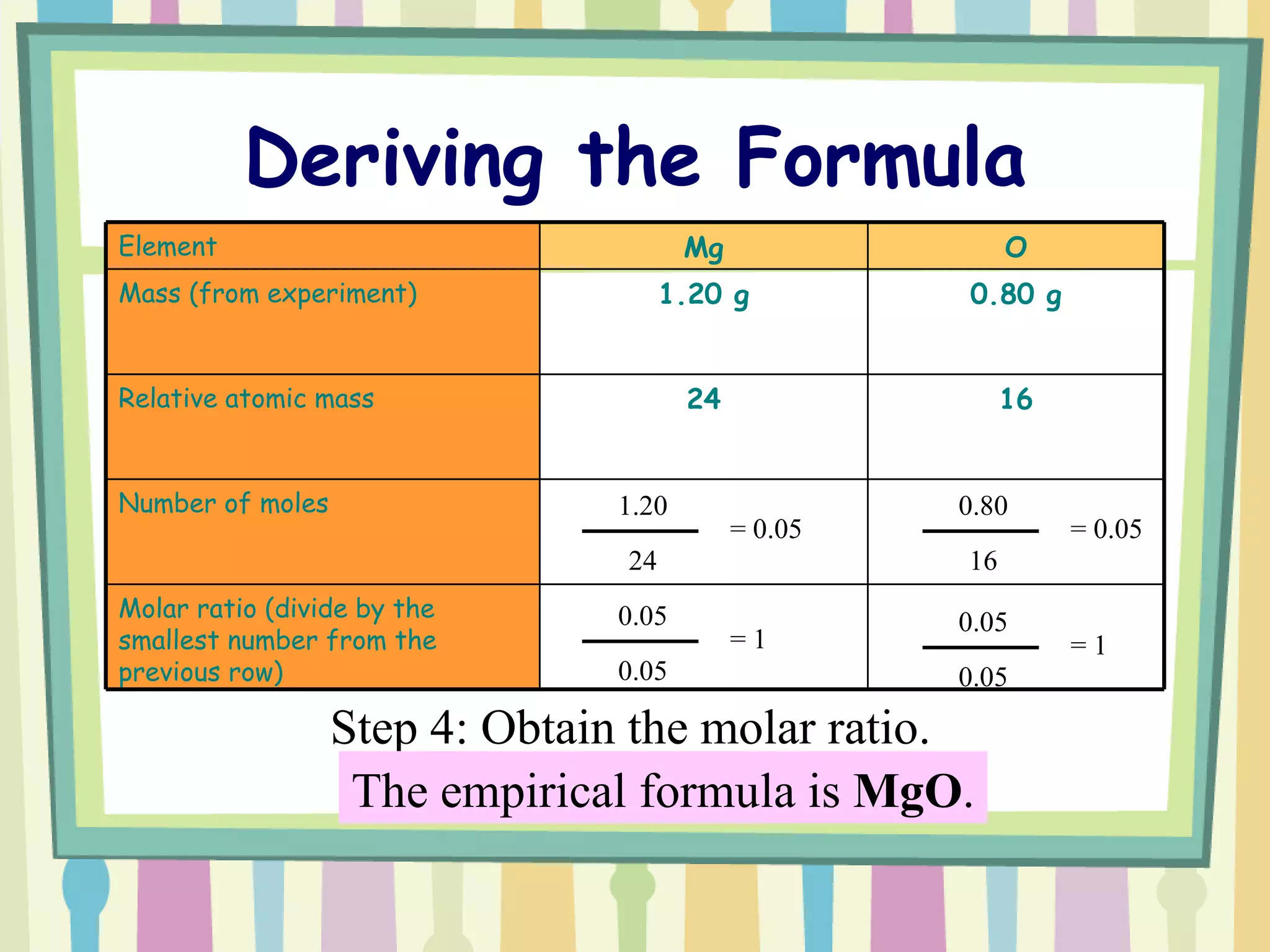
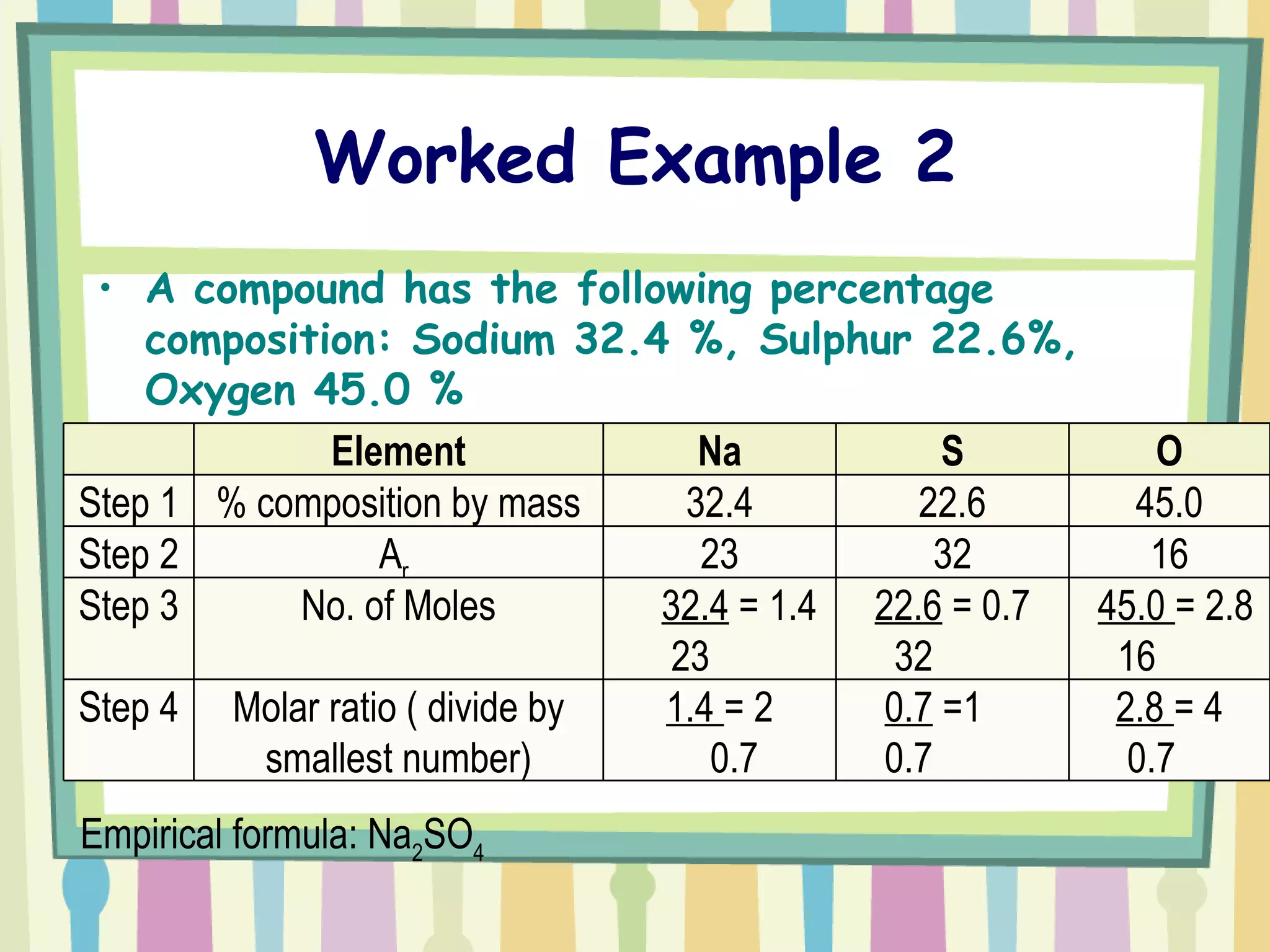
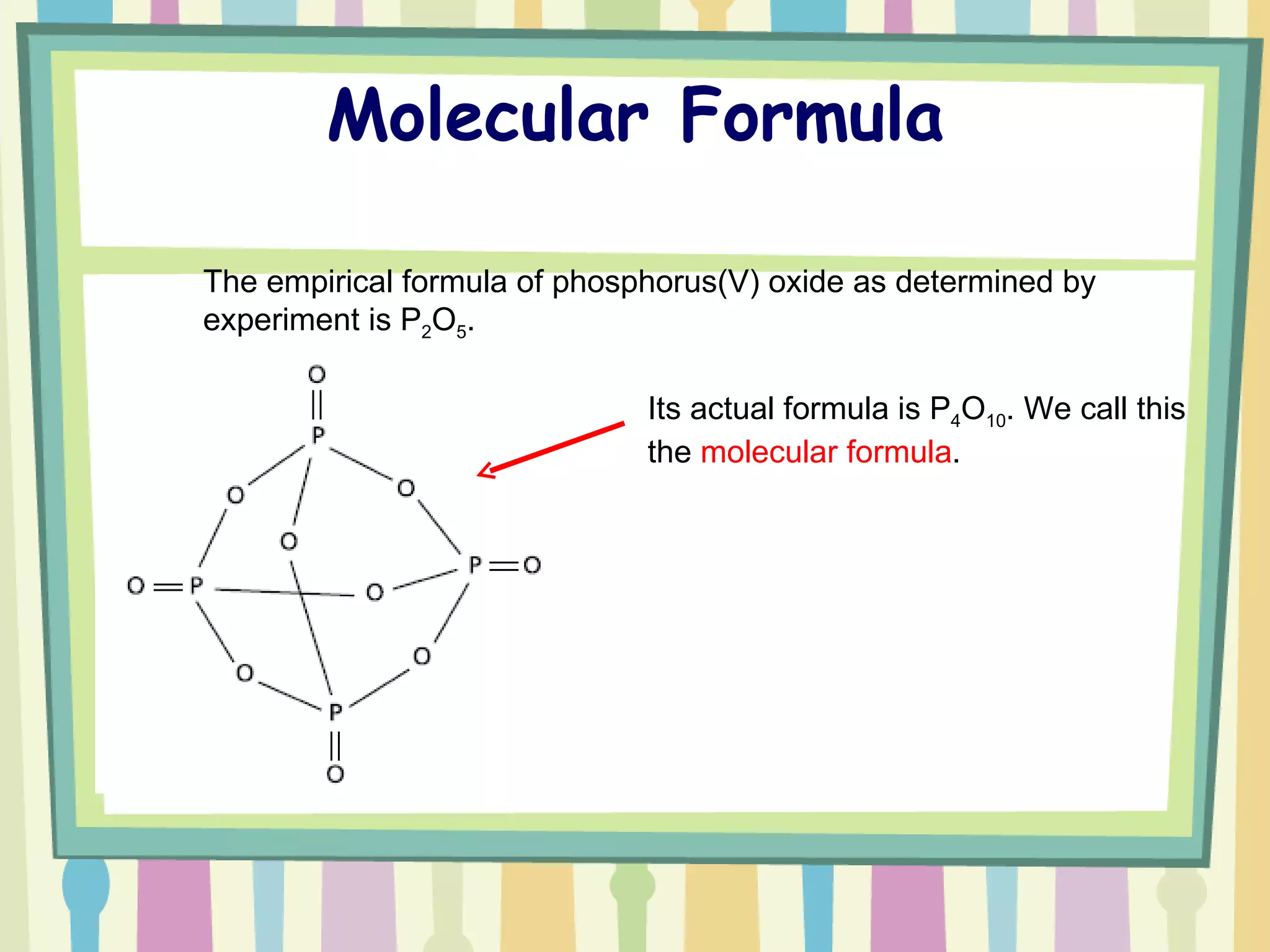
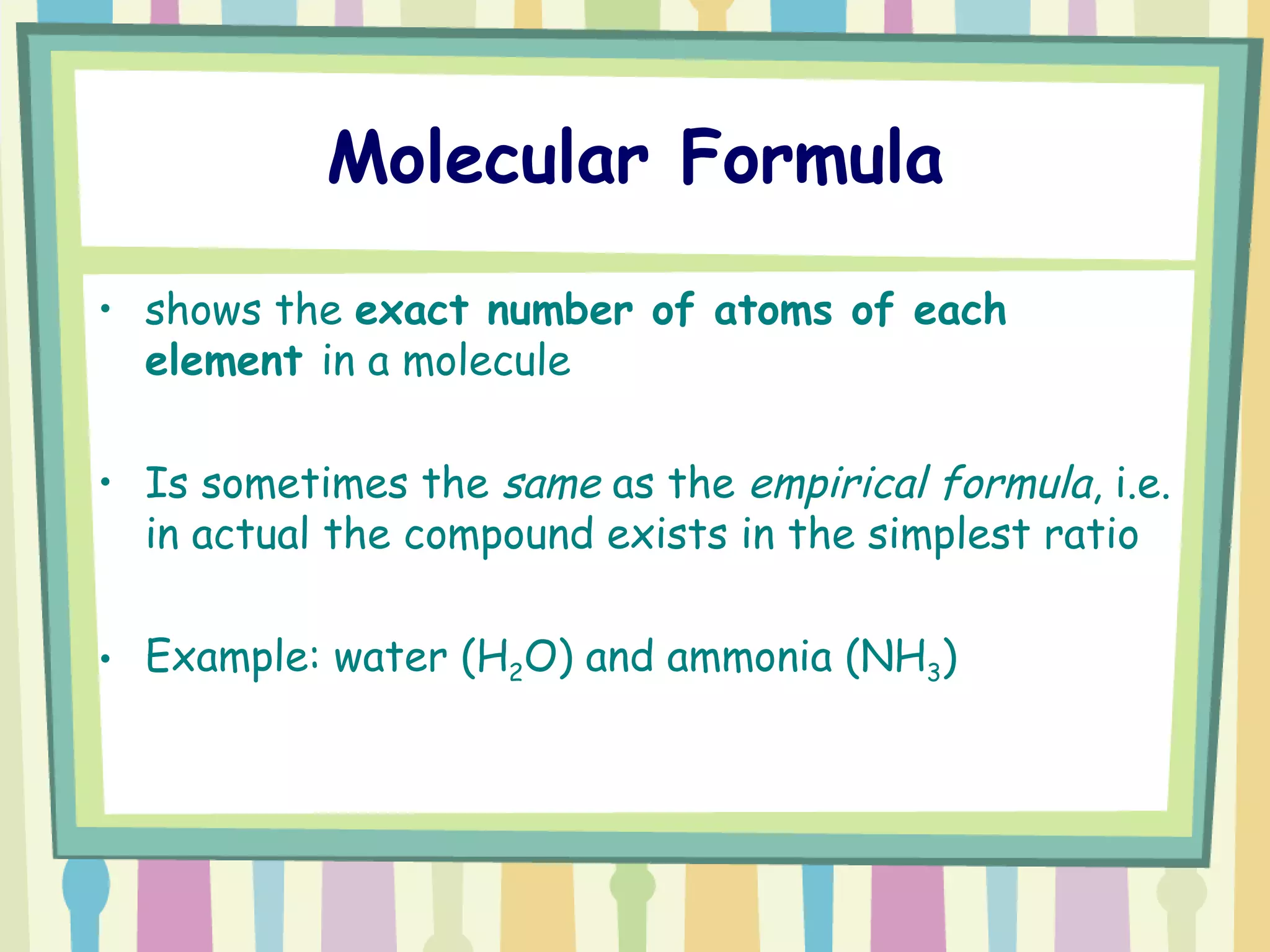
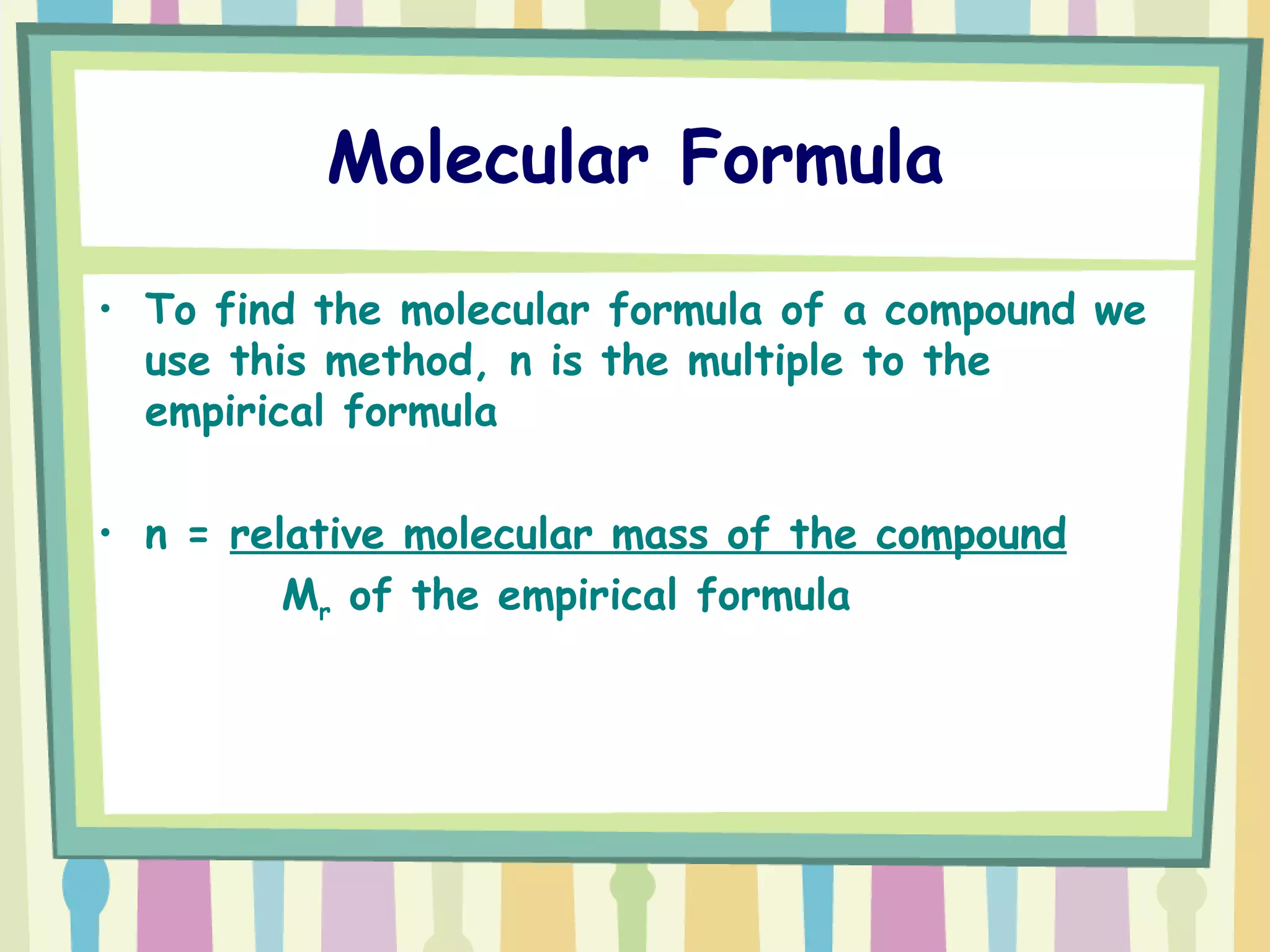
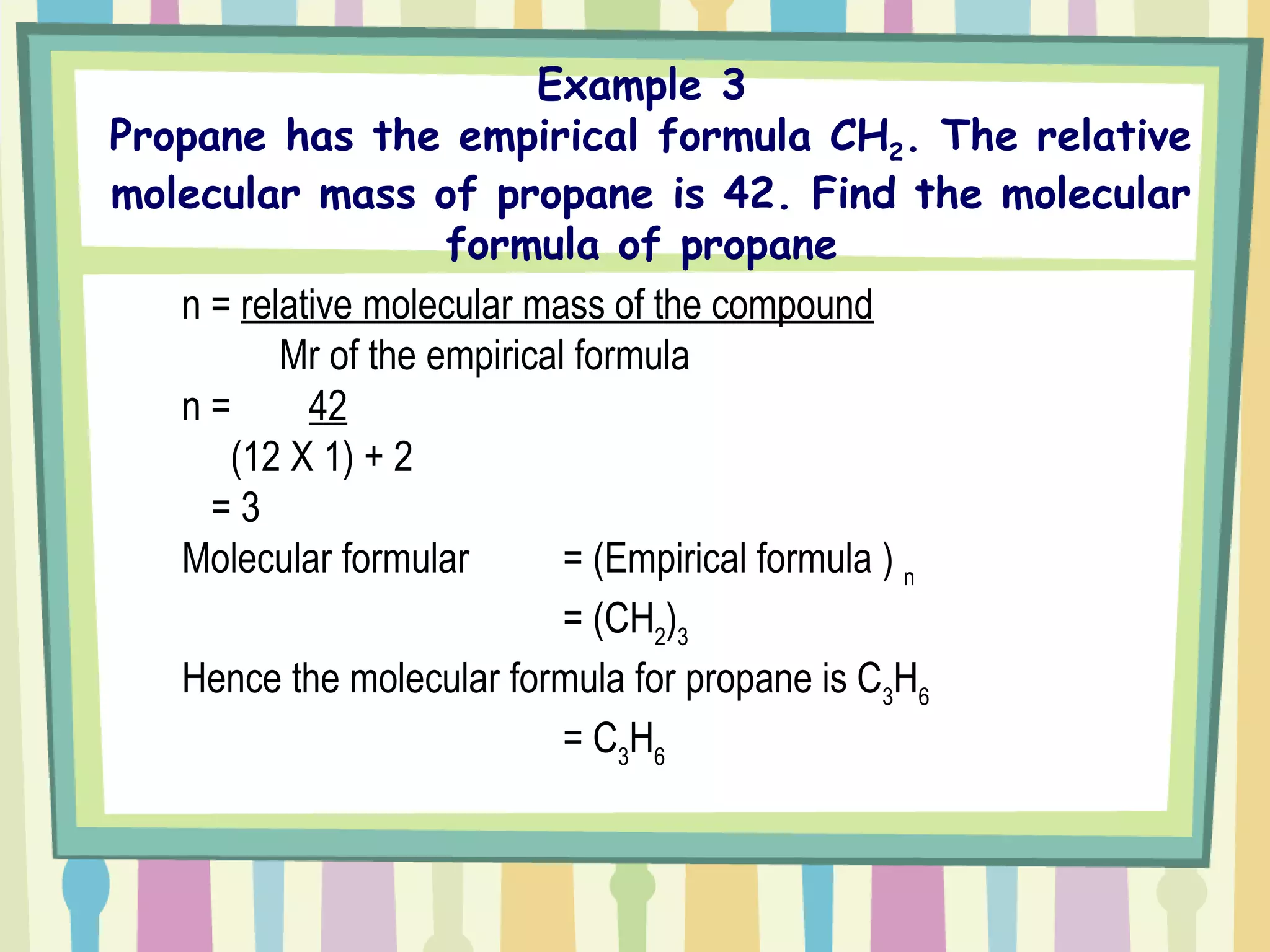
The document discusses how to determine the formula of a compound through experimentation. It provides an example of finding the formula of magnesium oxide. Key steps include measuring the masses of reactants and products, calculating moles of each substance, and determining the ratio of elements in the compound based on the mole ratios. The formula of magnesium oxide in this example is MgO.